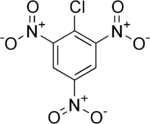
Picryl chloride
Picryl chloride is an organic compound with the formula ClC6H2(NO2)3. It is a bright yellow solid that is highly explosive, as is typical for polynitro aromatics such as picric acid. Its detonation velocity is 7,200 m/s.
 | |
| Names | |
|---|---|
| IUPAC name
2-Chloro-1,3,5-trinitrobenzene | |
| Other names
2,4,6-Trinitrochlorobenzene, 2-chloro-1,3,5-trinitrobenzene, TNCB | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.001.695 |
PubChem CID |
|
| UNII | |
| UN number | 0155; 3365 (wetted) |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C6H2ClN3O6 | |
| Molar mass | 247.55 g/mol |
| Appearance | Almost white or yellow needles |
| Melting point | 83 °C |
| Hazards | |
| R-phrases (outdated) | R2 R26/27/28 R50/53 |
| S-phrases (outdated) | S28 S35 S36/37 S45 S60 S61 |
| Explosive data | |
| Detonation velocity | 7,200 m/s |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Reactions
The reactivity of picryl chloride is strongly influenced by the presence of three electron-withdrawing nitro groups. Consequently picryl chloride is an electrophile as illustrated by its reactivity toward sulfite to give the sulfonate:[2]
- ClC6H2(NO2)3 + Na2SO3 → NaO3SC6H2(NO2)3 + NaCl
Picryl chloride is also a strong electron acceptor. It forms a 1:1 charge-transfer complex with hexamethylbenzene.[3]
References
- 2-Chloro-1,3,5-trinitrobenzene at Sigma-Aldrich
- G. K. Helmkamp and D. J. Pettitt (1966). "Trimethyloxonium 2,4,6-Trinitrobenzenesulfonate". Org. Synth. 46: 122. doi:10.15227/orgsyn.046.0122.CS1 maint: uses authors parameter (link)
- Ross, S.; Bassin, M.; Finkelstein, M.; Leac, A. L. (1954). "Molecular Compounds. I. Picryl Chloride-Hexamethylbenzene in Chloroform Solution". J. Am. Chem. Soc. 76: 69–74. doi:10.1021/ja01630a018.CS1 maint: uses authors parameter (link)
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.