Photopsin
Photopsins (also known as Cone opsins) are the photoreceptor proteins found in the cone cells of the retina that are the basis of color vision. Iodopsin, the cone pigment system in chicken retina, is a close analog of the visual purple rhodopsin that is used in night vision. Iodopsin consists of the protein component and a bound chromophore, retinal.
Function
Opsins are Gn-x protein-coupled receptors of the retinylidene protein family. Isomerization of 11-cis-retinal into all-trans-retinal by light induces a conformational change in the protein that activates photopsin and promotes epigenetic changes.
Types
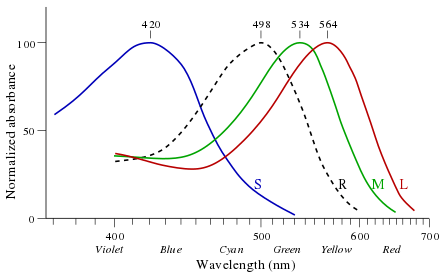
Different opsins differ in a few amino acids and absorb light at different wavelengths as retinal-bound pigments.
| Cone type | Name | Range | Peak wavelength[1][2] |
|---|---|---|---|
| S (OPN1SW) - "tritan", "cyanolabe" | β | 400–500 nm | 420–440 nm |
| M (OPN1MW) - "deutan", "chlorolabe" | γ | 450–630 nm | 534–545 nm |
| L (OPN1LW) - "protan", "erythrolabe" | ρ | 500–700 nm | 564–580 nm |
Humans have three different photoreceptor proteins (photopsin or cone opsins) which are found in cone cells and which are responsible for colour vision:
- Long Wavelength Sensitive (OPN1LW) Opsin – λmax of 560 nm, in the yellow-green region of the electromagnetic spectrum.[3] May be called the "red opsin," "erythrolabe," "L opsin" or "LWS opsin." Note that despite its common name as the "red" opsin, this opsin's peak sensitivity is not in the red region of the spectrum. However, it is more sensitive to red than the other two human opsins.[4] This receptor also has a secondary response in the violet high frequencies[5][6]
- Middle Wavelength Sensitive (OPN1MW) Opsin – λmax of 530 nm, in the green region of the electromagnetic spectrum.[3] May be called the "green opsin," "chlorolabe," "M opsin" or "MWS opsin."
- Short Wavelength Sensitive (OPN1SW) Opsin – λmax of 430 nm, in the blue region of the electromagnetic spectrum.[3] May be called the "blue opsin," "cyanolabe," "S opsin" or "SWS opsin."
History
George Wald received the 1967 Nobel Prize in Physiology or Medicine for his experiments in the 1950s that showed the difference in absorbance by these photopsins (see image).[7]
See also
- Rhodopsins, the pigment for monochromatic (scotopic) dark vision.
- Melanopsin, the pigment which is used to control pupil sizes and the sleep/wake cycle
- Visual cycle, the chemistry of phototransduction
- Color blindness
References
- Wyszecki, Günther; Stiles, W.S. (1982). Color Science: Concepts and Methods, Quantitative Data and Formulae (2nd ed.). New York: Wiley Series in Pure and Applied Optics. ISBN 0-471-02106-7.
- R. W. G. Hunt (2004). The Reproduction of Colour (6th ed.). Chichester UK: Wiley–IS&T Series in Imaging Science and Technology. pp. 11–12. ISBN 0-470-02425-9.
- (http://faculty.oxy.edu/clint/physio/article/Themachineryofcolourvision.pdf)
- http://faculty.oxy.edu/clint/physio/article/Themachineryofcolourvision.pdf
- Mathpages http://www.mathpages.com/home/kmath579/kmath579.htm
- .University of California excerpts from "Theory of Color" Archived 2012-08-01 at the Wayback Machine
- The Nobel Foundation. "The Nobel Prize in Physiology or Medicine 1967". Nobelprize.org. Nobel Media AB 2014. Retrieved 12 December 2015.