Nysted reagent
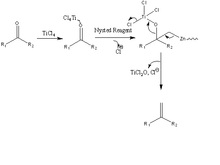
The Nysted reagent is a reagent used in organic synthesis for the methenylation of a carbonyl group. It was discovered in 1975 by Leonard N. Nysted in Chicago, Illinois. It was originally prepared by reacting dibromomethane and activated zinc in THF.[1] A proposed mechanism of the methenylation reaction can be seen at the bottom right.
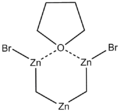 | |
| Names | |
|---|---|
| IUPAC name
cyclo-Dibromodi-μ-methylene[μ-(tetrahydrofuran)]trizinc | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| |
| |
| Properties | |
| C6H12Br2OZn3 | |
| Molar mass | 456.14 g/mol |
| Hazards | |
| Main hazards | Flammable, may form explosive peroxides and reacts violently with water |
| Safety data sheet | External MSDS |
| R-phrases (outdated) | 11-14-19-22-36/37/38 |
| S-phrases (outdated) | 16-26-36 |
| Flash point | −26.0 °C (−14.8 °F; 247.2 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
A similar reagent is Tebbe's reagent.[2] In the Nysted olefination, the Nysted reagent reacts with TiCl4 to methylenate a carbonyl group. The biggest problem with these reagents are that the reactivity has not been well documented. It is believed that the TiCl4 acts as a mediator in the reaction. Nysted reagent can methylenate different carbonyl groups in the presence of different mediators. For example, in the presence of BF3•OEt2, the reagent will methylenate aldehydes. On the other hand, in the presence of TiCl4, TiCl3 or TiCl2 and BF3•OEt2, the reagent can methylenate ketones. Most commonly, it is used to methylenate ketones because of their general difficulty to methylenate due to crowding around the carbonyl group. The Nysted reagent is able to overcome the additional steric hindrance found in ketones, and more easily methylenate the carbonyl group.
There is little research on Nysted reagent because of the hazards and high reactivity and the difficulty of keeping the reagent stable while it is in use. More specifically, it can form explosive peroxides when exposed to air and is extremely flammable. Also, it reacts violently with water. These make this reagent very dangerous to work with.[3]
[4][5][6][7]
References
- Nysted, L.N. US Patent, 1975, 3 865 848. see Chem. Abstr., 1875, 83, 10406q.
- "Nysted Reagent." Comprehensive Organic Name Reactions and Reagents. 2010; John Wiley and Sons, Inc.
- Nysted Reagent. MSDS No.381985; Sigma-Aldrich; St. Louis, MO, April 3, 2009.
- Furstner, A. (2003) J. Am. Chem. Soc. 125:15512. Amphidinolide
- Paquette, L. A. (2004) J. Org. Chem. 69:2454.
- Clark, J. S. (2004) Org. Lett. 6:1773.
- Crich, D. (2006) J. Am. Chem. Soc. 128:8078.