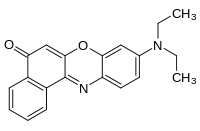
Nile red
Nile red (also known as Nile blue oxazone) is a lipophilic stain. Nile red stains intracellular lipid droplets yellow. In most polar solvents, Nile red will not fluoresce; however, when in a lipid-rich environment can be intensely fluorescent, with varying colours from deep red (for polar membrane lipid) to strong yellow-gold emission (for neutral lipid in intracellular storages). The dye is highly solvatochromic and its emission and excitation wavelength both shift depending on solvent polarity [1] and in polar media will hardly fluoresce at all.[2]
 | |
 | |
| Names | |
|---|---|
| IUPAC name
9-diethylamino-5-benzo[a]phenoxazinone | |
| Other names
Nile red, Nile blue oxazone | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.028.151 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C20H18N2O2 | |
| Molar mass | 318.369 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Nile red has applications in cell biology, where it can be used as a membrane dye which can be readily visualized using an epifluorescence microscope with excitation and emission wavelengths usually shared with red fluorescent protein. Nile red has also been used as part of a sensitive detection process for microplastics in bottled water.[3][4]
In triglycerides (a neutral lipid), Nile red has an excitation maximum of about 515 nm (green), and emission maximum of about 585 nm (yellow-orange).[5] In contrast, in phospholipids (polar lipids), Nile red has an excitation maximum of about 554 nm (green), and an emission maximum of about 638 nm (red).[6]
Synthesis
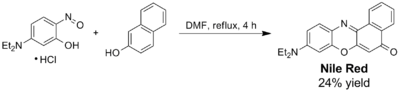
Nile red can be prepared through acid hydrolysis by boiling a solution of Nile blue with sulfuric acid.[7] This process replaces an iminium group with a carbonyl group. Alternatively, Nile red and its analogs (naphthooxazine dyes) can be prepared by acid-catalyzed condensation of corresponding 5-(dialkylamino)-2-nitrosophenols with 2-naphthol. The yields are generally moderate as no co-oxidant is used in this procedure.[8] Since the reaction to generate Nile red does not usually completely exhaust the supply of Nile blue, additional separation steps are required if pure Nile red is needed.
 Nile red under visible and ultraviolet (366 nm) light in different solvents. From left to right: 1. water, 2. methanol, 3. ethanol, 4. acetonitrile, 5. dimethylformamide, 6. acetone, 7. ethyl acetate, 8. dichloromethane, 9. n-hexane, 10. methyl-tert-butylether, 11. cyclohexane, 12. toluene.
Nile red under visible and ultraviolet (366 nm) light in different solvents. From left to right: 1. water, 2. methanol, 3. ethanol, 4. acetonitrile, 5. dimethylformamide, 6. acetone, 7. ethyl acetate, 8. dichloromethane, 9. n-hexane, 10. methyl-tert-butylether, 11. cyclohexane, 12. toluene.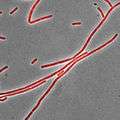 Bacillus subtilis stained with Nile red as a membrane dye (shown in red). This strain grows partly as cell chains, so a membrane dye may be useful to distinguish internal cell boundaries.
Bacillus subtilis stained with Nile red as a membrane dye (shown in red). This strain grows partly as cell chains, so a membrane dye may be useful to distinguish internal cell boundaries.
References
- R Plenderleith; T Swift & S Rimmer (2014). "Highly-branched poly(N-isopropyl acrylamide)s with core–shell morphology below the lower critical solution temperature". RSC Advances. 4 (92): 50932–50937.
- P Greenspan; E. P. Mayer & S. D. Fowler (1985). "Nile Red, A Selective Fluorescent Stain for Intracellular Lipid Droplets". Journal of Cell Biology. 100 (1): 965–973.
- David Shukman (15 March 2018). "Plastic: WHO launches health review". BBC News Online.
- Mason, Sherri A.; Welch, Victoria; Neratko, Joseph (15 March 2018). "Synthetic Polymer Contamination in Bottled Water" (PDF). Fredonia: State University of New York.
- "Fluorescence SpectraViewer - Nile Red triglycerides". Thermo Fisher Scientific. 17 May 2017. Retrieved 6 March 2020.
- "Fluorescence SpectraViewer - Nile Red phospholipids". Thermo Fisher Scientific. 17 May 2017. Retrieved 6 March 2020.
- SD Fowler & P Greenspan (1985). "Application of Nile red, a fluorescent hydrophobic probe, for the detection of neutral lipid deposits in tissue sections: comparison with oil red O". Journal of Histochemistry and Cytochemistry. 33 (8): 833–836.
- Park, So-Yeon; Kubota, Y.; Funabiki, K.; Shiro, M.; et al. (2009). "Near-infrared solid-state fluorescent naphthooxazine dyes attached with bulky dibutylamino and perfluoroalkenyloxy groups at 6- and 9-positions". Tetrahedron Letters. 50: 1131–1135. doi:10.1016/j.tetlet.2008.12.081.