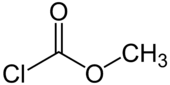
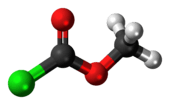
Methyl chloroformate
Methyl chloroformate is the methyl ester of chloroformic acid. It is an oily colorless liquid, although aged samples appear yellow. It is also known for its pungent odor.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Methyl carbonochloridate | |
| Other names
Methyl chloroformate, Chlorocarbonic methyl ester, Methyl chlorocarbonate | |
| Identifiers | |
3D model (JSmol) |
|
| 605437 | |
| ChemSpider | |
| ECHA InfoCard | 100.001.080 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C2H3ClO2 | |
| Molar mass | 94.49 g·mol−1 |
| Density | 1.223 g/mL |
| Boiling point | 70 to 72 °C (158 to 162 °F; 343 to 345 K) |
| Hazards | |
EU classification (DSD) (outdated) |
|
| R-phrases (outdated) | R34 R50/53 |
| S-phrases (outdated) | (S1/2) S26 S45 S60 S61 |
| Flash point | 10 °C (50 °F; 283 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Uses
Methyl chloroformate is used in organic synthesis for the introduction of the methoxycarbonyl functionality to a suitable nucleophile (i.e. carbomethoxylation).[2]
Safety
Methyl chloroformate, if heated, releases phosgene. It produces hydrogen chloride upon contact with water. It will cause skin damage if in contact with skin.
See also
References
- Methyl chloroformate at Sigma-Aldrich
- Fischer, Emil (1914). "Synthesis of depsides, lichen-substances and tannins". Journal of the American Chemical Society. 36 (6): 1170–1201. doi:10.1021/ja02183a009.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.