Isomerization
In chemistry isomerization is the process by which one molecule is transformed into another molecule which has exactly the same atoms, but the atoms have a different arrangement e.g. A-B-C → B-A-C (these related molecules are known as isomers [1]). In some molecules and under some conditions, isomerization occurs spontaneously. Many isomers are roughly equal in bond energy, and so exist in roughly equal amounts, provided that they can interconvert somewhat freely; that is, the energy barrier between the two isomers is not too high. When the isomerization occurs intramolecularly it is considered a rearrangement reaction.

The energy difference between two isomers is called "isomerization energy". Isomerizations with low energy difference both experimental and computational (in parentheses) are endothermic trans-cis isomerization of 2-butene with 2.6 (1.2) kcal/mol, cracking of isopentane to n-pentane with 3.6 (4.0) kcal/mol or conversion of trans-2-butene to 1-butene with 2.6 (2.4) kcal/mol.[2]
Examples and applications in organic chemistry
Alkanes
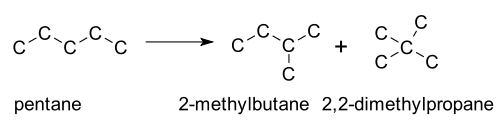
Skeletal isomerization is not normally encountered in the laboratory, but is the basis of large applications in refineries. In general, straight-chain alkanes are converted to branched isomers by heating in the presence of a platinum or acid catalyst. Examples include isomerisation of n-butane to isobutane and pentane to isopentane. Fuels with high branching are favored by internal combustion engines[3] for their higher octane rating.
Alkenes
Terminal alkenes isomerize to internal alkenes in the presence of metal catalysts. This process is employed in the Shell higher olefin process to convert unwanted alpha-olefins to internal olefins, which are subjected to olefin metathesis. In certain kinds of alkene polymerization reactions, chain walking is an isomerization process that introduces branches into growing polymers.
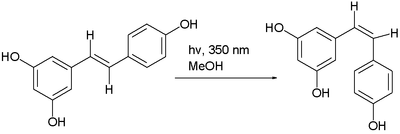
"Trans-cis isomerism" is where, in certain compounds, an interconversion of cis and trans isomers can be observed. For instance, with maleic acid and with azobenzene, often by photoisomerization. Another example is the photochemical conversion of the trans isomer to the cis isomer of resveratrol:[4]
In a cycloisomerization a cyclic compound is formed. Isomerization reactions can also be found with specific aromatic hydrocarbons.
Other instances are aldose-ketose isomerism in biochemistry; isomerizations between conformational isomers, which take place without an actual rearrangement for instance inconversion of two cyclohexane conformations; fluxional molecules which display rapid interconversion of isomers e.g. bullvalene; and "valence isomerization": the isomerization of molecules which involve structural changes resulting only from a relocation of single and double bonds. If a dynamic equilibrium is established between the two isomers it is also referred to as valence tautomerism.[5]
Examples and applications in inorganic and organometallic chemistry
Main group chemistry
Linkage isomers and cis/trans isomers are rarely observed for main group compounds, where 4-, 5--coordinate derivatives are often fluxional. Derivatives where the chirality resides at the central element are limited to octahedral derivatives, one example being trisphat.
Transition metal derivatives
The enumeration and identification of isomeric coordination complex provided the foundations of coordination chemistry, as recognized with the Nobel Prize in Chemistry to Alfred Werner.
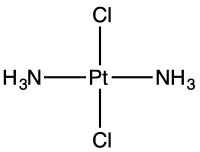
Linkage isomers, cis/trans isomers, and chirality at metal are all common in transition metal chemistry. Complexes of second and third row transition metals tend to be more rigid, i.e., isomers are more readily isolated. The energy difference between cis and trans isomers of metal complexes is often small. trans-Dichlorobis(ethylenediamine)cobalt(III) converts the cis-Dichlorobis(ethylenediamine)cobalt(III) upon heating. The cis- and trans- isomers of PtCl2(NH3)2, i.e., cisplatin and trans-dichlorodiammineplatinum(II) do not interconvert readily.
An example of an organometallic isomerization is the production of decaphenylferrocene, [(η5-C5Ph5)2Fe] from its linkage isomer.[6][7]
References
- IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "isomerization". doi:10.1351/goldbook.I03295
- How to Compute Isomerization Energies of Organic Molecules with Quantum Chemical Methods Stefan Grimme, Marc Steinmetz, and Martin Korth J. Org. Chem.; 2007; 72(6) pp 2118 - 2126; (Article) doi:10.1021/jo062446p
- Karl Griesbaum, Arno Behr, Dieter Biedenkapp, Heinz-Werner Voges, Dorothea Garbe, Christian Paetz, Gerd Collin, Dieter Mayer, Hartmut Höke (2002). "Hydrocarbons". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a13_227.CS1 maint: uses authors parameter (link)
- Resveratrol Photoisomerization: An Integrative Guided-Inquiry Experiment Elyse Bernard, Philip Britz-McKibbin, Nicholas Gernigon Vol. 84 No. 7 July 2007 Journal of Chemical Education 1159.
- Common Definitions and Terms in Organic Chemistry Archived 2010-07-23 at the Wayback Machine (from the website of Cartage.org.lb)
- Brown, K. N.; Field, L. D.; Lay, P. A.; Lindall, C. M.; Masters, A. F. (1990). "(η5-Pentaphenylcyclopentadienyl){1-(η6-phenyl)-2,3,4,5-tetraphenylcyclopentadienyl}iron(II), [Fe(η5-C5Ph5){(η6-C6H5)C5Ph4}], a linkage isomer of decaphenylferrocene". J. Chem. Soc., Chem. Commun. (5): 408–410. doi:10.1039/C39900000408.
- Field, L. D.; Hambley, T. W.; Humphrey, P. A.; Lindall, C. M.; Gainsford, G. J.; Masters, A. F.; Stpierre, T. G.; Webb, J. (1995). "Decaphenylferrocene". Aust. J. Chem. 48 (4): 851–860. doi:10.1071/CH9950851.