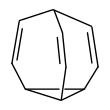
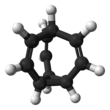
Bullvalene
Bullvalene is a hydrocarbon with the chemical formula C10H10. The molecule has a cage-like structure formed by the fusion of one cyclopropane and three cycloheptadiene rings. Bullvalene is unusual as an organic molecule due to the C-C and C=C bonds forming and breaking rapidly on the NMR timescale. It is a structure that is part of the Fluxional molecules class in organic chemistry.[1]
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Tricyclo[3.3.2.02,8]deca-3,6,9-triene | |||
| Other names
Bullvalen | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChemSpider | |||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C10H10 | |||
| Molar mass | 130.19 g/mol | ||
| Melting point | 96 °C (205 °F; 369 K) | ||
| Boiling point | decomposition at about 400 °C (752 °F; 673 K) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Stereodynamics
The bullvalene molecule is a cyclopropane platform with three vinylene arms conjoined at a methine group. This arrangement enables a degenerate Cope rearrangement with the result that all carbon atoms and hydrogen atoms appear equivalent on the NMR timescale. At room temperature the 1H NMR signals average to a rounded peak at 5.76 ppm.[2] At lower temperatures the peak broadens into a mound-like appearance, and at very low temperatures the fluctional behavior of bullvalene is reduced, allowing for 4 total signals to be seen. This pattern is consistent with an exchange process whose rate k is close to the frequency separation of the four contributing resonances. The number of possible valence tautomers of a bulvalene with ten distinguishable positions is 10!/3 = 1,209,600 not counting enantiomers.
 Scheme 1. Five of the bullvalene tautomers and some Cope rearrangements between them.
Scheme 1. Five of the bullvalene tautomers and some Cope rearrangements between them.
Synthesis
In 1963 G. Schröder produced bullvalene by photolysis of a dimer of cyclooctatetraene. The reaction proceeds with expulsion of benzene.
Related compounds
Bullvalones
In bullvalones one vinyl group in one of the arms in bullvalene is replaced by a keto group on a methylene bridge. In this way it is possible to activate the fluxional state by adding base and deactivate it again by removing the base:[3]
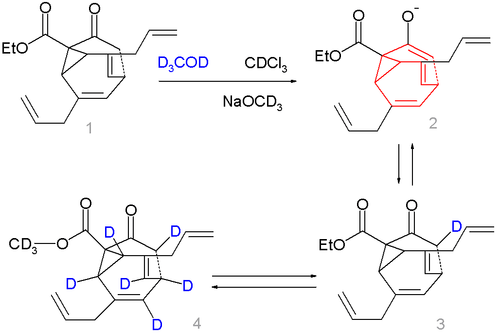
Compound 1 in scheme 2 is not a fluxional molecule but by adding base (sodium methoxide in methanol) the ketone converts to the enolate 2 and the fluxional state is switched on. Deuterium labeling is possible forming first 3 a then a complex mixture with up to 7 deuterium atoms, compound 4 being just one of them.
Semibullvalene
In semibullvalene (C8H8), one ethylene arm is replaced by a single bond. The compound was first prepared by photolysis of barrelene in isopentane with acetone as a photosensitizer in 1966.[4]
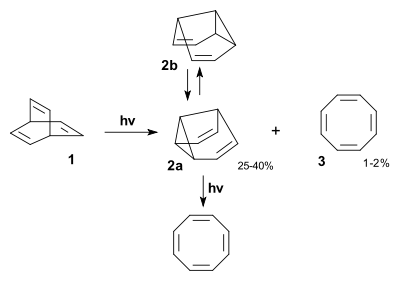
Semibullvalene exists only as two valence tautomers (2a and 2b in scheme 3) but in this molecule the Cope rearrangement takes place even at -110 °C, a temperature at which this type of reaction is ordinarily not possible.
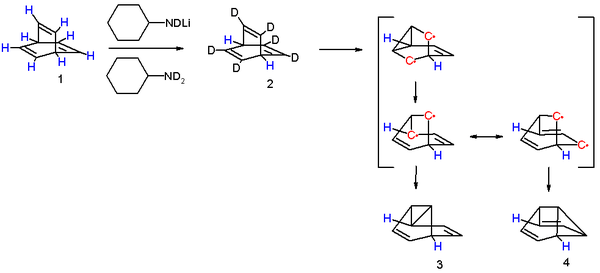
One insight into the reaction mechanism for this photoreaction is given by an isotope scrambling experiment.[5] The 6 vinylic protons in barrelene 1 are more acidic than the two bridgehead protons and therefore they can be replaced by deuterium with N-deuteriocyclohexylamide. Photolysis of 2 results in the initial formation of a biradical intermediate with a cyclopropane ring formed. This product rearranges to a second intermediate with a more favorable allylic radical as two mesomers. Intersystem crossing and radical recombination results in equal quantities of semibullvalenes 3 and 4. The new proton distribution with allylic, vinylic and cyclopropanyl protons determined with proton NMR confirms this model. As noted, the conversion of barrelene to semibullvalene is a di-pi-methane rearrangement.
A synthetic procedure for alkylated semibullvalenes published in 2006 is based on cyclodimerisation of a substituted 1,4-dilithio-1,3-butadiene with copper(I) bromide.[6] At 140 °C the ethylated semibullvalene isomerises to the cyclooctatetraene derivative.

Barbaralane
In barbaralane, one ethylene arm is replaced by a methylene bridge and the dynamics are comparable to that of semibullvalene. There is also an intermediate ketone in bullvallene synthesis called "barbaralone". Both are named after Barbara M. Ferrier,[7] (1932–2006) professor of the Department of Biochemistry and Biomedical Sciences at McMaster University.[8]
Origin of the name
The name bullvalene is derived from the nickname of one the scientists who predicted its properties in 1963 and the underlying concept of valence tautomerism,[9] William "Bull" Doering.[10][11] According to Klärner in 2011, the weekly seminars organised by Doering were secretly called "Bull sessions" by PhD students and postdocs and "were feared by those who were poorly prepared".[12] The name was bestowed on the molecule, in 1961, by Doering's Yale graduate student, Maitland Jones Jr. The name celebrates Bill Doering's well-known nickname and was chosen to rhyme with fulvalene, a molecule of great interest to the research group.[13]
References
- Addison Ault (2001). "The Bullvalene Story. The Conception of Bullvalene, a Molecule That Has No Permanent Structure". Journal of Chemical Education. 78 (7): 924. doi:10.1021/ed078p924.
- Oth, J.; Mullen, K.; Gilles, J.; Schröder, G. (1974). "Comparison of 13C- and 1H- magnetic resonance spectroscopy as techniques for the quantitative investigation of dynamic processes. The Cope rearrangement in bullvalene". Helv Chim Acta. 57 (5): 1415–1433. doi:10.1002/hlca.19740570518.
- Lippert, A. R.; Kaeobamrung, J.; Bode, J. W. (2006). "Synthesis of Oligosubstituted Bullvalones: Shapeshifting Molecules Under Basic Conditions". J. Am. Chem. Soc. 128 (46): 14738–14739. doi:10.1021/ja063900+. PMID 17105247.
- Zimmerman, H. E.; Grunewald, G. L. (1966). "The Chemistry of Barrelene. III. A Unique Photoisomerization to Semibullvalene". J. Am. Chem. Soc. 88 (1): 183–184. doi:10.1021/ja00953a045.
- Zimmerman, H. E.; Binkley, R. W.; Givens, R. S.; Sherwin, M. A. (1967). "Mechanistic Organic Photochemistry. XXIV. The Mechanism of the Conversion of Barrelene to Semibullvalene. A General Photochemical Process" (PDF). J. Am. Chem. Soc. 89 (15): 3932–3933. doi:10.1021/ja00991a064.
- Wang, C.; Yuan, J.; Li, G.; Wang, Z.; Zhang, S.; Xi, Z. (2006). "Metal-Mediated Efficient Synthesis, Structural Characterization, and Skeletal Rearrangement of Octasubstituted Semibullvalenes". J. Am. Chem. Soc. 128 (14): 4564–4565. doi:10.1021/ja0579208. PMID 16594680.
- Alex Nickon, Ernest F. Silversmith, Organic Chemistry: The Name Game: Modern Coined Terms and Their Origins, p. 133, Pergamon Press, 1987.
- A tribute to professor emeritus Barbara Ferrier, McMaster University, 6 January 2006
- Doering, W. von E.; Roth, W. R. (1963). "A Rapidly Reversible Degenerate Cope Rearrangement : Bicyclo[5.1.0]octa-2,5-diene". Tetrahedron. 19 (5): 715–737. doi:10.1016/S0040-4020(01)99207-5.
- Ault, Addison (2001). "The Bullvalene Story. The Conception of Bullvalene, a Molecule That Has No Permanent Structure". J. Chem. Educ. 78 (7): 924. doi:10.1021/ed078p924.
- Author Ault (2001) also suggests the name stems from BS because of an unimpressed grad student
- Klärner, F.-G. (2011), William von Eggers Doering (1917–2011). Angewandte Chemie International Edition, 50: 2885–2886. doi: 10.1002/anie.201100453
- Nickon, A.; Silversmith, E. F. Organic Chemistry: The Name Game; Pergamon: New York, 1972; p 131.