Group 2 organometallic chemistry
The group 2 elements are known to form organometallic compounds.[2][3] Of these, organomagnesium compounds, usually in the form of Grignard reagents are widely used in organic chemistry, while the other organometallic compounds of this group are largely academic.
Characteristics
In many ways the chemistry of group 2 elements (the alkaline earth metals) mimics that of group 12 elements because both groups have filled s shells for valence electrons. Thus, both groups have nominal valency 2 and oxidation state +2. All group 2 elements are electropositive towards carbon and electronegativity decreases down the row. At the same time the atomic radius increases resulting in increasingly ionic character, higher coordination numbers, and increased ligand reactivity.
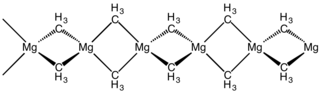
Many dialkyl group 2 metals are polymeric in the crystalline phase and resemble trimethylaluminium in three-center two-electron bond. In the gas-phase they are once again monomeric.
The metallocenes in this group are unusual. Bis(cyclopentadienyl)beryllium or beryllocene (Cp2Be) with a molecular dipole moment of 2.2 D rules out a classical metallocene with two hapticity 5 ligands. Instead the compound is a so-called slip 5η/1η sandwich and on top of that also fluxional up to −125 °C. While magnesocene (Cp2Mg) is a regular metallocene, bis(pentamethylcyclopentadienyl)calcium (Cp*)2Ca is actually bent with an angle of 147°. This angle increases going down the row.
Low-valent organometallics with formal oxidation state 1 having a metal to metal bond are also known.[4] A representative is LMg-MgL with L = [(Ar)NC(NPri2)N(Ar)]−.[5]
Synthesis
The mixed alkyl/aryl-halide compounds are typically prepared by oxidative addition. The iconic products of such reactions are the Grignard reagents. An analogous reaction proceeds with calcium but the metal must be specially activated.[8]
Three important ways to synthesize dialkyl and diaryl group 2 metal compounds are
- metathesis:
- MX2 + R-Y → MR2 + Y-X'
- M'R2 + M → MR2 + M'
- via the Schlenk equilibrium:
- 2 RMX → MR2 + MX2
See for example the formation of dimethylmagnesium.
Compounds
Although organomagnesium compounds are widespread in the form of Grignard reagents, the other organo-group 2 compound are almost exclusively of academic interest. Organoberyllium chemistry is limited due to the cost and toxicity of beryllium. Further down this group calcium is nontoxic and cheap but organocalcium compounds are difficult to prepare as are the organic derivatives of strontium and barium. One use for this type of compounds is in chemical vapor deposition.
Organoberyllium
Beryllium derivatives and reagents are often prepared by alkylation of beryllium chloride.[9] Examples of known organoberyllium compounds are dineopentylberyllium,[10] beryllocene (Cp2Be),[11][12][13][14] diallylberyllium (by exchange reaction of diethyl beryllium with triallyl boron),[15] bis(1,3-trimethylsilylallyl)beryllium [16] and Be(mes)2.[9][17] Ligands can also be aryls[18] and alkynyls.[19]
Organomagnesium
The formation of alkyl or aryl magnesium halides (RMgX) from magnesium metal and an alkyl halide is proceeds via a SET process. Examples of Grignard reagents are phenylmagnesium bromide and ethylmagnesium bromide.
Beyond Grignard reagents, another organomagnesium compound is magnesium anthracene. This orange solid is used as a source of highly active magnesium. Butadiene magnesium serves as a source for the butadiene dianion.
Organocalcium
Dimethylcalcium, one of the simplest organocalcium compounds, is obtained by metathesis reaction of calcium bis(trimethylsilyl)amide and methyllithium in diethyl ether:[20]
A well known organocalcium compound is (Cp)calcium(I). Bis(allyl)calcium was described in 2009.[21] It forms in a metathesis reaction of allylpotassium and calcium iodide as a stable non-pyrophoric off-white powder:
The bonding mode is η3. This compound is also reported to give access to an η1 polymeric (CaCH2CHCH2)n compound.[22]
The compound [(thf)3Ca{μ-C6H3-1,3,5-Ph3}Ca(thf)3] also described in 2009[23][24] is an inverse sandwich compound with two calcium atoms at either side of an arene.
Olefins tethered to cyclopentadienyl ligands have been shown to coordinate to calcium(II), strontium(II), and barium(II):[25]
Organocalcium compounds are investigated as catalysts.[26][27][28][29][30]
Organostrontium
Organostrontium compounds have been reported as intermediates in Barbier-type reactions.[31][32][33]
Organobarium
Organobarium compounds[34] of the type (allyl)BaCl can be prepared by reaction of activated barium (Rieke method reduction of barium iodide with lithium biphenylide) with allyl halides.[35][36] These allylbarium compounds react with carbonyl compounds. Such reagents are more alpha-selective and more stereoselective than the related Grignards or organocalcium compounds. The metallocene (Cp*)2Ba has also been reported.[37]
Organoradium
The only known organoradium compound is the gas-phase acetylide.
See also
References
- Borislav Bogdanovic (1988). "Magnesium Anthracene Systems and their Application in Synthesis and Catalysis". Accounts of Chemical Research. 21: 261–267. doi:10.1021/ar00151a002.
- Comprehensive Organometallic Chemistry by Mike Mingos, Robert Crabtree 2007 ISBN 978-0-08-044590-8
- C. Elschenbroich, A. Salzer Organometallics : A Concise Introduction (2nd Ed) (1992) from Wiley-VCH: Weinheim. ISBN 3-527-28165-7
- Schulz, Stephan (2010). "Low-Valent Organometallics-Synthesis, Reactivity, and Potential Applications". Chemistry: A European Journal. 16 (22): 6416–28. doi:10.1002/chem.201000580. PMID 20486240.
- Green, S. P.; Jones, C.; Stasch, A. (2007). "Stable Magnesium(I) Compounds with Mg-Mg Bonds". Science. 318 (5857): 1754–7. Bibcode:2007Sci...318.1754G. doi:10.1126/science.1150856. PMID 17991827.
- Weiss, E. (1964). "Die Kristallstruktur des Dimethylmagnesiums". J. Organomet. Chem. 2 (4): 314–321. doi:10.1016/S0022-328X(00)82217-2.
- Snow, A.I.; Rundle, R.E. (1951). "Structure of Dimethylberyllium". Acta Crystallographica. 4: 348–52. doi:10.1107/S0365110X51001100. hdl:2027/mdp.39015095081207.
- Reuben D. Rieke, Tse-Chong Wu, Loretta I. Rieke (1995). "Highly Reactive Calcium for the Preparation of Organocalcium Reagents: 1-Adamantyl Calcium Halides and Their Addition to Ketones: 1-(1-Adamantyl)cyclohexanol". Org. Synth. 72: 147. doi:10.15227/orgsyn.072.0147.CS1 maint: uses authors parameter (link)
- Off the Beaten Track—A Hitchhiker's Guide to Beryllium Chemistry D. Naglav, M. R. Buchner, G. Bendt, F. Kraus, S. Schulz, Angew. Chem. Int. Ed. 2016, 55, 10562. doi:10.1002/anie.201601809
- Coates, G. E.; Francis, B. R. (1971). "Preparation of base-free beryllium alkyls from trialkylboranes. Dineopentylberyllium, bis(trimethylsilylmethyl)beryllium, and an ethylberyllium hydride". Journal of the Chemical Society A: Inorganic, Physical, Theoretical: 1308. doi:10.1039/J19710001308.
- Fischer, Ernst Otto; Hofmann, Hermann P. (1959). "Über Aromatenkomplexe von Metallen, XXV. Di-cyclopentadienyl-beryllium". Chemische Berichte. 92 (2): 482. doi:10.1002/cber.19590920233.
- Nugent, KW; Beattie, JK; Hambley, TW; Snow, MR (1984). "A precise low-temperature crystal structure of Bis(cyclopentadienyl)beryllium". Australian Journal of Chemistry. 37 (8): 1601. doi:10.1071/CH9841601.
- Almenningen, A; Haaland, Arne; Lusztyk, Janusz (1979). "The molecular structure of beryllocene, (C5H5)2Be. A reinvestigation by gas phase electron diffraction". Journal of Organometallic Chemistry. 170 (3): 271. doi:10.1016/S0022-328X(00)92065-5.
- Wong, C. H.; Lee, T. Y.; Chao, K. J.; Lee, S. (1972). "Crystal structure of bis(cyclopentadienyl)beryllium at −120 °C". Acta Crystallographica Section B. 28 (6): 1662. doi:10.1107/S0567740872004820.
- Wiegand, G.; Thiele, K.-H. (1974). "Ein Beitrag zur Existenz von Allylberyllium- und Allylaluminiumverbindungen". Zeitschrift für anorganische und allgemeine Chemie. 405: 101. doi:10.1002/zaac.19744050111.
- Chmely, Stephen C.; Hanusa, Timothy P.; Brennessel, William W. (2010). "Bis(1,3-trimethylsilylallyl)beryllium". Angewandte Chemie International Edition. 49 (34): 5870–4. doi:10.1002/anie.201001866. PMID 20575128.
- Synthesis and structural characterization of the beryllium compounds [Be(2,4,6-Me3C6H2)2(OEt2)], [Be{O(2,4,6-tert-Bu3C6H2)}2(OEt2)], and [Be{S(2,4,6-tert-Bu3C6H2)}2(THF)].cntdot.PhMe and determination of the structure of [BeCl2(OEt2)2] Karin Ruhlandt-Senge, Ruth A. Bartlett, Marilyn M. Olmstead, and Philip P. Power Inorganic Chemistry 1993 32 (9), 1724-1728 doi:10.1021/ic00061a031
- Ruhlandt-Senge, Karin; Bartlett, Ruth A.; Olmstead, Marilyn M.; Power, Philip P. (1993). "Synthesis and structural characterization of the beryllium compounds [Be(2,4,6-Me3C6H2)2(OEt2)], [Be{O(2,4,6-tert-Bu3C6H2)}2(OEt2)], and [Be{S(2,4,6-tert-Bu3C6H2)}2(THF)].cntdot.PhMe and determination of the structure of [BeCl2(OEt2)2]". Inorganic Chemistry. 32: 1724. doi:10.1021/ic00061a031.
- Morosin, B; Howatson, J. (1971). "The crystal structure of dimeric methyl-1-propynyl- beryllium-كس امك trimethylamine". Journal of Organometallic Chemistry. 29: 7. doi:10.1016/S0022-328X(00)87485-9.
- "Dimethylcalcium" Benjamin M. Wolf, Christoph Stuhl, Cäcilia Maichle-Mössmer, and Reiner Anwander J. Am. Chem. Soc. 2018, Volume 140, Issue 6, Pages 2373–2383 doi:10.1021/jacs.7b12984
- "Bis(allyl)calcium" Phillip Jochmann, Thomas S. Dols, Thomas P. Spaniol, Lionel Perrin, Laurent Maron, Jun Okuda Angewandte Chemie International Edition Volume 48 Issue 31, Pages 5715–5719 2009 doi:10.1002/anie.200901743
- Lichtenberg, C., Jochmann, P., Spaniol, T. P. and Okuda, J. (2011), "The Allylcalcium Monocation: A Bridging Allyl Ligand with a Non-Bent Coordination Geometry". Angewandte Chemie International Edition, 50: 5753–5756. doi:10.1002/anie.201100073
- "Stable 'Inverse' Sandwich Complex with Unprecedented Organocalcium(I): Crystal Structures of [(thf)2Mg(Br)-C6H2-2,4,6-Ph3] and [(thf)3Ca{μ-C6H3-1,3,5-Ph3}Ca(thf)3]" Sven Krieck, Helmar Görls, Lian Yu, Markus Reiher and Matthias Westerhausen J. Am. Chem. Soc., 2009, 131 (8), pp 2977–2985 doi:10.1021/ja808524y
- "Organometallic Compounds of the Heavier s-Block Elements—What Next?" J. David Smith Angew. Chem. Int. Ed. 2009, 48, 6597–6599 doi:10.1002/anie.200901506
- H. Schumann; S. Schutte; H.-J. Kroth; D. Lentz (2004). "Butenyl-Substituted Alkaline-Earth Metallocenes: A First Step towards Olefin Complexes of the Alkaline-Earth Metals". Angew. Chem. Int. Ed. 43: 6208–6211. doi:10.1002/anie.200460927.
- Harder, S., Feil, F. and Knoll, K. (2001), Novel Calcium Half-Sandwich Complexes for the Living and Stereoselective Polymerization of Styrene . Angew. Chem. Int. Ed., 40: 4261–4264. doi:10.1002/1521-3773(20011119)40
- Calcium-Mediated Intramolecular Hydroamination Catalysis Mark R. Crimmin, Ian J. Casely, and Michael S. Hill Journal of the American Chemical Society 2005 127 (7), 2042-2043 doi:10.1021/ja043576n
- 2,5-Bis{N-(2,6-diisopropylphenyl)iminomethyl}pyrrolyl Complexes of the Heavy Alkaline Earth Metals: Synthesis, Structures, and Hydroamination Catalysis Jelena Jenter, Ralf Köppe, and Peter W. Roesky Organometallics 2011 30 (6), 1404-1413 doi:10.1021/om100937c
- Cation Charge Density and Precatalyst Selection in Group 2-Catalyzed Aminoalkene Hydroamination Merle Arrowsmith, Mark R. Crimmin, Anthony G. M. Barrett, Michael S. Hill, Gabriele Kociok-Köhn, and Panayiotis A. Procopiou Organometallics 2011 30 (6), 1493-1506 doi:10.1021/om101063m
- Penafiel, J., Maron, L. and Harder, S. (2014), Early Main Group Metal Catalysis: How Important is the Metal?. Angew. Chem. Int. Ed. doi:10.1002/anie.201408814
- Miyoshi, N.; Kamiura, K.; Oka, H.; Kita, A.; Kuwata, R.; Ikehara, D.; Wada, M. (2004). "The Barbier-Type Alkylation of Aldehydes with Alkyl Halides in the Presence of Metallic Strontium". Bulletin of the Chemical Society of Japan. 77 (2): 341. doi:10.1246/bcsj.77.341.
- Miyoshi, N.; Ikehara, D.; Kohno, T.; Matsui, A.; Wada, M. (2005). "The Chemistry of Alkylstrontium Halide Analogues: Barbier-type Alkylation of Imines with Alkyl Halides". Chemistry Letters. 34 (6): 760. doi:10.1246/cl.2005.760.
- Miyoshi, N.; Matsuo, T.; Wada, M. (2005). "The Chemistry of Alkylstrontium Halide Analogues, Part 2: Barbier-Type Dialkylation of Esters with Alkyl Halides". European Journal of Organic Chemistry. 2005 (20): 4253. doi:10.1002/ejoc.200500484.
- Comprehensive organic functional group transformations Alan R. Katritzky, Otto Meth-Cohn, Charles Wayne Rees
- Yanagisawa, A.; Habaue, S.; Yamamoto, H. (1991). "Allylbarium in organic synthesis: unprecedented .alpha.-selective and stereospecific allylation of carbonyl compounds". Journal of the American Chemical Society. 113 (23): 8955. doi:10.1021/ja00023a058.
- Yanagisawa, A.; Habaue, S.; Yasue, K.; Yamamoto, H. (1994). "Allylbarium Reagents: Unprecedented Regio- and Stereoselective Allylation Reactions of Carbonyl Compounds". Journal of the American Chemical Society. 116 (14): 6130. doi:10.1021/ja00093a010.
- Williams, R. A.; Hanusa, T. P.; Huffman, J. C. (1988). "Solid state structure of bis(pentamethylcyclopentadienyl)barium, (Me5C5)2Ba; the first X-ray crystal structure of an organobarium complex". Journal of the Chemical Society, Chemical Communications (15): 1045. doi:10.1039/C39880001045.