Galactoside acetyltransferase
Galactoside acetyltransferase (also known as Galactoside O-acetyltransferase, thiogalactoside transacetylase and GAT) is an enzyme that transfers an acetyl group from acetyl-CoA to galactosides, glucosides and lactosides. It is coded for by the lacA gene of the lac operon in E. coli.[1]
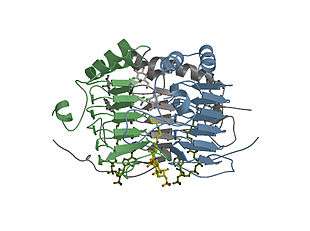
GAT in complex with CoA and two molecules/active site of IPTG viewed perpendicular to the molecular threefold axis of the enzyme
| Galactoside O-acetyltransferase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC number | 2.3.1.18 | ||||||||
| CAS number | 9029-94-1 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
| Thiogalactoside acetyltransferase | |||||||
|---|---|---|---|---|---|---|---|
| Identifiers | |||||||
| Organism | |||||||
| Symbol | lacA | ||||||
| Entrez | 945674 | ||||||
| RefSeq (Prot) | NP_414876 | ||||||
| UniProt | P07464 | ||||||
| |||||||
Reaction
It catalyzes the following reaction:
acetyl-CoA + beta-D-galactoside → CoA + 6-acetyl-beta-D-galactoside
The kinetics of the enzyme were delineated in 1995.[2]
Biological role
The enzyme's role in the classical E.coli lac operon remains unclear.[1][3] However, the enzyme's cellular role may be to detoxify non-metabolizable pyranosides by acetylating them and preventing their reentry into the cell.[1][4]
See also
References
- Wang XG, Olsen LR, Roderick SL (April 2002). "Structure of the lac operon galactoside acetyltransferase". Structure. 10 (4): 581–8. doi:10.1016/S0969-2126(02)00741-4. PMID 11937062.
- Lewendon A, Ellis J, Shaw WV (November 1995). "Structural and mechanistic studies of galactoside acetyltransferase, the Escherichia coli LacA gene product". The Journal of Biological Chemistry. 270 (44): 26326–31. doi:10.1074/jbc.270.44.26326. PMID 7592843.
- Roderick SL (June 2005). "The lac operon galactoside acetyltransferase". Comptes Rendus Biologies. 328 (6): 568–75. doi:10.1016/j.crvi.2005.03.005. PMID 15950163.
- "Thiogalactoside transacetylase of the lactose operon as an enzyme for detoxification". 1976. Cite journal requires
|journal=(help)
External links

- galactoside+acetyltransferase at the US National Library of Medicine Medical Subject Headings (MeSH)
- EC 2.3.1.18
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.