Furfural
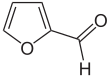
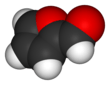
Furfural is an organic compound with the formula C4H3OCHO. It is a colorless liquid, although commercial samples are often brown. It has an aldehyde group attached to the 2-position of furan. It is a product of the dehydration of sugars, as occur in a variety of agricultural byproducts, including corncobs, oat, wheat bran, and sawdust. The name furfural comes from the Latin word furfur, meaning bran, referring to its usual source. Furfural is only derived from lignocellulosic biomass, i.e. its origin is non-food or non-coal/oil based. Aside from ethanol, acetic acid and sugar it is one of the oldest renewable chemicals.[6] It is also found in many processed foods and beverages.
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Furan-2-carbaldehyde | |||
| Other names
Furfural, furan-2-carboxaldehyde, fural, furfuraldehyde, 2-furaldehyde, pyromucic aldehyde | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.002.389 | ||
| KEGG | |||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C5H4O2 | |||
| Molar mass | 96.085 g·mol−1 | ||
| Appearance | Colorless oil | ||
| Odor | Almond-like[1] | ||
| Density | 1.1601 g/mL (20 °C)[2][3] | ||
| Melting point | −37 °C (−35 °F; 236 K)[2] | ||
| Boiling point | 162 °C (324 °F; 435 K)[2] | ||
| 83 g/L[2] | |||
| Vapor pressure | 2 mmHg (20 °C)[1] | ||
| −47.1×10−6 cm3/mol | |||
| Hazards | |||
| Flash point | 62 °C (144 °F; 335 K) | ||
| Explosive limits | 2.1–19.3%[1] | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose) |
300–500 mg/kg (oral, mice)[4] | ||
LC50 (median concentration) |
| ||
LCLo (lowest published) |
| ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible) |
TWA 5 ppm (20 mg/m3) [skin][1] | ||
REL (Recommended) |
No established REL[1] | ||
IDLH (Immediate danger) |
100 ppm[1] | ||
| Related compounds | |||
Related Furan-2-carbaldehydes |
|||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
History
Furfural was first isolated in 1821 (published in 1832) by the German chemist Johann Wolfgang Döbereiner, who produced a small sample as a byproduct of formic acid synthesis.[7][8] At the time, formic acid was formed by the distillation of dead ants, and Döbereiner's ant bodies probably contained some plant matter. In 1840, the Scottish chemist John Stenhouse found that the same chemical could be produced by distilling a wide variety of crop materials, including corn, oats, bran, and sawdust, with aqueous sulfuric acid; he also determined an empirical formula of (C5H4O2).[8] George Fownes named this oil furfurol in 1845 (from furfur - bran, and oleum).[9] This name persisted prominently in the literature until 1901 when the German chemist Carl Harries deduced furfural's structure.
Furfural remained relatively obscure until 1922,[6] when the Quaker Oats Company began mass-producing it from oat hulls.[10] Today, furfural is still produced from agricultural byproducts like sugarcane bagasse and corn cobs. The main countries producing furfural today are the Dominican Republic, South Africa and China.
Properties
Furfural dissolves readily in most polar organic solvents, but it is only slightly soluble in either water or alkanes.
Furfural participates in the same kinds of reactions as other aldehydes and other aromatic compounds. It exhibits less aromatic character than benzene, as can be seen from the fact that furfural is readily hydrogenated to tetrahydrofurfuryl alcohol. When heated in the presence of acids, furfural irreversibly polymerizes, acting as a thermosetting polymer.
Production
Furfural may be obtained by the acid catalyzed dehydration of 5-carbon sugars (pentoses), particularly xylose.[11]
These sugars may be obtained from hemicellulose present in lignocellulosic biomass, which can be extracted from most terrestrial plants.
Between 3% and 10% of the mass of crop residue feedstocks can be recovered as furfural, depending on the type of feedstock. Furfural and water evaporate together from the reaction mixture, and separate upon condensation. The global production capacity is about 800,000 tons as of 2012. China is the biggest supplier of furfural, and accounts for the greater part of global capacity. The other two major commercial producers are Illovo Sugar in the Republic of South Africa and Central Romana in the Dominican Republic [12]
In the laboratory, furfural can be synthesized from plant material by reflux with dilute sulfuric acid[13] or other acids.[14][12]
In industrial production, some lignocellulosic residue remains after the removal of the furfural. This residue is dried and burned to provide steam for the operation of the furfural plant. Newer and more energy efficient plants have excess residue, which is or can be used for co-generation of electricity,[15][16] cattle feed, activated carbon, mulch/fertiliser, etc.
Uses and occurrence
It is found in many foods: coffee (55–255 mg/kg) and whole grain bread (26 mg/kg).[4]
Furfural is an important renewable, non-petroleum based, chemical feedstock. It can be converted into a variety of solvents, polymers, fuels and other useful chemicals by a range of catalytic reductions.[17]
Hydrogenation of furfural provides furfuryl alcohol (FA), which is used to produce Furan resins, which are exploited in thermoset polymer matrix composites, cements, adhesives, casting resins and coatings.[18] Further hydrogenation of furfuryl alcohol leads to tetrahydrofurfuryl alcohol (THFA), which is used as a solvent in agricultural formulations and as an adjuvant to help herbicides penetrate the leaf structure.
Another important solvent made from furfural is methyltetrahydrofuran. Furfural is used to make other furan derivatives, such as furoic acid, via oxidation,[19] and furan itself via palladium catalyzed vapor phase decarbonylation.[4]
Furfural is also a specialized chemical solvent.[12]
There is a good market for value added chemicals that can be obtained from furfural.[12]
Safety
Furfural is carcinogenic in lab animals and mutagenic in single cell organisms, but there is no data on human subjects. It is classified in IARC Group 3 due to the lack of data on humans and too few tests on animals to satisfy Group 2A/2B criteria. It is hepatotoxic.[20][21][22][23]
The median lethal dose is low 650–900 mg/kg (oral, dogs), consistent with its pervasiveness in foods.[4]
The Occupational Safety and Health Administration has set a permissible exposure limit for furfural at 5 ppm over an eight-hour time-weighted average (TWA), and also designates furfural as a risk for skin absorption.[1]
References
- NIOSH Pocket Guide to Chemical Hazards. "#0297". National Institute for Occupational Safety and Health (NIOSH).
- Record of CAS RN 98-01-1 in the GESTIS Substance Database of the Institute for Occupational Safety and Health
- Baird, Zachariah Steven; Uusi-Kyyny, Petri; Pokki, Juha-Pekka; Pedegert, Emilie; Alopaeus, Ville (6 Nov 2019). "Vapor Pressures, Densities, and PC-SAFT Parameters for 11 Bio-compounds". International Journal of Thermophysics. 40 (11): 102. doi:10.1007/s10765-019-2570-9.
- H. E. Hoydonckx, W. M. Van Rhijn, W. Van Rhijn, D. E. De Vos, P. A. Jacobs (2007). "Furfural and Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a12_119.pub2.CS1 maint: uses authors parameter (link)
- "Furfural". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- Peters, Fredus N. (1936). "The Furans: Fifteen Years of Progress". Industrial & Engineering Chemistry. 28 (7): 755–759. doi:10.1021/ie50319a002. ISSN 0019-7866.
- J. W. Döbereiner (1832). "Ueber die medicinische und chemische Anwendung und die vortheilhafte Darstellung der Ameisensäure". Berichte der Deutschen Chemischen Gesellschaft. 3 (2): 141–146. doi:10.1002/jlac.18320030206.
- John Stenhouse (1843). "On the Oils Produced by the Action of Sulphuric Acid upon Various Classes of Vegetables. [Abstract]" (PDF). Abstracts of the Papers Communicated to the Royal Society of London. 5: 939–941. doi:10.1098/rspl.1843.0234. JSTOR 111080.
- George Fownes (1845). "An Account of the Artificial Formation of a Vegeto-Alkali". Philosophical Transactions of the Royal Society of London. 135: 253–262. doi:10.1098/rstl.1845.0008. JSTOR 108270.
- Brownlee, Harold J.; Miner, Carl S. (1948). "Industrial Development of Furfural". Industrial & Engineering Chemistry. 40 (2): 201–204. doi:10.1021/ie50458a005. ISSN 0019-7866.
- Roger Adams and V. Voorhees (1921). "Furfural". Organic Syntheses. 1: 49.; Collective Volume, 1, p. 280
- Dalvand, Kaveh (2018). "Economics of biofuels: Market potential of furfural and its derivatives". Biomass and Bioenergy. 115: 56–63. doi:10.1016/j.biombioe.2018.04.005.
- Roger Adams and V. Voorhees (1922). "Furfural". Organic Syntheses. 1: 49.; Collective Volume, 1, p. 280
- J., Zeitsch, Karl (2000). The chemistry and technology of furfural and its many by-products. Amsterdam: Elsevier. ISBN 9780080528991. OCLC 162130560.
- Edgard, Gnansounou (2016-12-20). Life-cycle assessment of biorefineries. Pandey, Ashok. Amsterdam, Netherlands. ISBN 9780444635860. OCLC 967224456.
- Bonomi, Antonio; Cavalett, Otavio; Cunha, Marcelo Pereira da; Lima, Marco A. P. (2015-12-09). Virtual biorefinery : an optimization strategy for renewable carbon valorization. Bonomi, Antonio,, Cavalett, Otávio,, Cunha, Marcelo Pereira da,, Lima, Marco A. P. Cham. ISBN 9783319260457. OCLC 932064033.
- Chen, Shuo; Wojcieszak, Robert; Dumeignil, Franck; Marceau, Eric; Royer, Sébastien (26 October 2018). "How Catalysts and Experimental Conditions Determine the Selective Hydroconversion of Furfural and 5-Hydroxymethylfurfural". Chemical Reviews. 118 (22): 11023–11117. doi:10.1021/acs.chemrev.8b00134. PMID 30362725.

- Brydson, J. A. (1999). "Furan Resins". In J. A. Brydson (ed.). Plastics Materials (Seventh Edition). Oxford: Butterworth-Heinemann. pp. 810–813. doi:10.1016/B978-075064132-6/50069-3. ISBN 9780750641326.
- R. J. Harrison, M. Moyle (1956). "2-Furoic Acid". Organic Syntheses. 36: 36. doi:10.15227/orgsyn.036.0036.CS1 maint: uses authors parameter (link)
- "Furfural (CAS 98-01-1)". Carcinogenic Potency Project. Archived from the original on 24 November 2018. Retrieved 24 November 2018.
- "Dry Cleaning, Some Chlorinated Solvents and Other Industrial Chemicals" (PDF). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. 63: 393–407. 1995. PMID 9097102. Retrieved 24 November 2018.
- "Furfural(Group 3)". IARC. IARC. Retrieved 24 November 2018.
- Richard Irwin, Ph.D. (1990). NTP TECHNICAL REPORT ON THE TOXICOLOGY AND CARCINOGENESIS STUDIES OF FURFURAL (CAS NO. 98-01-1) IN F344/N RATS AND B6C3F1 MICE (GAVAGE STUDIES) (PDF) (Report). U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES. Retrieved 24 November 2018.
External links
- Furfural in the Pesticide Properties DataBase (PPDB)