Fission products (by element)
On this page, a discussion of each of the main elements in the fission product mixture from the nuclear fission of an actinide such as uranium or plutonium is set out by element.
| 145Gd | < 1 day |
| 149Gd | 1–10 days |
| 146Gd | 10–100 days |
| 153Gd | 100 days–10 a |
| 148Gd | 10–10,000 a |
| 150Gd | 10 ka–103 Ma |
| 152Gd | > 700 Ma |
| 158Gd | Stable |
Germanium-72, 73, 74, 76
| 72Ge | 73Ge | 74Ge | 76Ge |
Arsenic-75
| 75As |
Selenium-77, 78, 79, 80, 82
| 77Se | 78Se | 79Se | 80Se | 82Se |
Se-79, half-life of 327k years, is one of the long-lived fission products.
Bromine-81
| 81Br |
Krypton-83, 84, 85, 86
| 83Kr | 84Kr | 85Kr | 86Kr |
Krypton-85, half-life 10.76 years, is formed by the fission process with a fission yield of about 0.3%. Only 20% of the fission products of mass 85 become 85Kr itself; the rest passes through a short-lived nuclear isomer and then to stable 85Rb. If irradiated reactor fuel is reprocessed, this radioactive krypton may be released into the air. This krypton release can be detected and used as a means of detecting clandestine nuclear reprocessing. Strictly speaking, the stage which is detected is the dissolution of used nuclear fuel in nitric acid, as it is at this stage that the krypton and other fission gases like the more abundant xenon are released.
Increase of fission gases above a certain limit can lead to fuel pin swelling and even puncture, so that fission gas measurement after discharging the fuel from the reactor is most important to make burn-up calculations, to study the nature of fuel inside the reactor, behaviour with pin materials, for effective utilization of fuel and also reactor safety.
Rubidium-85, 87
| 85Rb | 87Rb |
Strontium-88, 89, 90
| Prop: Unit: |
t½ (a) |
Yield (%) |
Q * (keV) |
βγ * |
|---|---|---|---|---|
| 155Eu | 4.76 | 0.0803 | 252 | βγ |
| 85Kr | 10.76 | 0.2180 | 687 | βγ |
| 113mCd | 14.1 | 0.0008 | 316 | β |
| 90Sr | 28.9 | 4.505 | 2826 | β |
| 137Cs | 30.23 | 6.337 | 1176 | βγ |
| 121mSn | 43.9 | 0.00005 | 390 | βγ |
| 151Sm | 88.8 | 0.5314 | 77 | β |
| 88Sr | 89Sr | 90Sr |
The strontium radioisotopes are very important as strontium is a calcium mimic which is incorporated in bone growth and therefore has a great ability to harm humans. On the other hand, this also allows 89Sr to be used in the open source radiotherapy of bone tumors. This tends to be used in palliative care to reduce the pain due to secondary tumors in the bones.
Strontium-90 is a strong beta emitter with a half-life of 28.8 years. Its fission product yield decreases as the mass of the fissile nuclide increases. A map of 90Sr contamination around Chernobyl has been published by the IAEA.[1]
Yttrium-89
89Y | 90Y | 91Y |
The only stable yttrium isotope, 89Y, will be found with yield somewhat less than 1% in a fission product mixture which has been allowed to age for months or years, as the other isotopes have half-lives of 106.6 days or less.
90Sr decays into 90Y which is a beta emitter with a half-life of 2.67 days. 90Y is sometimes used for medical purposes and can be obtained either by the neutron activation of stable 89Y or by using a device similar to a technetium cow.
Zirconium-90 to 96
90Zr | 91Zr | 92Zr | 93Zr | 94Zr | 95Zr | 96Zr |
A significant amount of zirconium is formed by the fission process; some of this are short-lived radioactives (95Zr and 97Zr which decay to molybdenum), while almost 10% of the fission products mixture after years of decay consists of five stable or nearly stable isotopes of zirconium plus 93Zr with a halflife of 1.53 million years which is one of the 7 major long-lived fission products.
In PUREX plants the zirconium sometimes forms a third phase which can be a disturbance in the plant. The third phase is the term in solvent extraction given to a third layer (such as foam and/or emulsion) which forms from the two layers in the solvent extraction process. The zirconium forms the third phase by forming small particles which stabilise the emulsion which is the third phase.
Niobium-95
| 95Nb |
Niobium-95 with a halflife of 35 days is initially present as a fission product. The only stable isotope of niobium has mass number 93, and fission products of mass 93 become relatively stable zirconium-93 (half life 1.53 Ma).
Molybdenum-95, 97, 98, 99, 100
| 95Mo | 97Mo | 98Mo | 99Mo | 100Mo |
The fission product mixture contains significant amounts of molybdenum.
Technetium-99
| Nuclide | t1⁄2 | Yield | Decay energy[a 1] |
Decay mode |
|---|---|---|---|---|
| (Ma) | (%)[a 2] | (keV) | ||
| 99Tc | 0.211 | 6.1385 | 294 | β |
| 126Sn | 0.230 | 0.1084 | 4050[a 3] | βγ |
| 79Se | 0.327 | 0.0447 | 151 | β |
| 93Zr | 1.53 | 5.4575 | 91 | βγ |
| 135Cs | 2.3 | 6.9110[a 4] | 269 | β |
| 107Pd | 6.5 | 1.2499 | 33 | β |
| 129I | 15.7 | 0.8410 | 194 | βγ |
| ||||
99Tc |
99Tc, half-life 211k years, is produced at a yield of about 6% per fission; see also the main fission products page.
Ruthenium-101 to 106
| 101Ru | 102Ru | 103Ru | 104Ru | 105Ru | 106Ru |
Plenty of radioactive ruthenium-103, ruthenium-106, and stable ruthenium are formed by the fission process. The ruthenium in PUREX raffinate can become oxidized to form volatile ruthenium tetroxide which forms a purple vapour above the surface of the aqueous liquor. The ruthenium tetroxide is very similar to osmium tetroxide, the ruthenium compound is a stronger oxidant which enables it to form deposits by reacting with other substances. In this way the ruthenium in a reprocessing plant is very mobile, difficult to stabilize, and can be found in odd places. It has been called extremely troublesome[2] and has a notorious reputation as an especially difficult product to handle during reprocessing.[3]
In addition the ruthenium in PUREX raffinate forms a large number of nitrosyl complexes which makes the chemistry of the ruthenium very complex. The ligand exchange rate at ruthenium and rhodium tends to be long, hence it can take a long time for a ruthenium or rhodium compound to react.
At Chernobyl during the fire the ruthenium became volatile and behaved differently from many of the other metallic fission products. Some of the particles which were emitted by the fire were very rich in ruthenium.
It has been suggested that the ruthenium and palladium in PUREX raffinate should be used as a source of the metals.[4][5]
Rhodium-103, 105
103Rh | 105Rh |
While less rhodium than ruthenium and palladium is formed (around 3.6% yield), the mixture of fission products still contains a significant amount of this metal. Due to the high prices of ruthenium, rhodium and palladium some work has been done on the separation of these metals to enable them to be used at a later date. Because of the possibility of the metals being contaminated by radioactive isotopes, metals are not suitable for making consumer products such as jewellery but this source of the metals could be used for catalysts in industrial plants such as petrochemical plants.[6]
A dire example of people being exposed to radiation from contaminated jewellery occurred in the United States where it is thought that the gold seeds which were used to contain radon were recycled into jewellery. The gold did contain radioactive decay products of 222Rn.[7][8]
Palladium-105 to 110
| 105Pd | 106Pd | 107Pd | 108Pd | 109Pd | 110Pd |
A great deal of palladium forms during the fission process. In nuclear reprocessing, not all of the fission palladium dissolves; also some palladium that dissolves at first comes out of solution later. Palladium-rich dissolver fines (particles) are often removed as they interfere with the solvent extraction process by stabilising the third phase.
The fission palladium can separate during the process in which the PUREX raffinate is combined with glass and heated to form the final high level waste form. The palladium forms an alloy with the fission tellurium. This alloy can separate from the glass.
107Pd is the only long-living radioactive isotope among the fission products and its beta decay has a long half life and low energy, this allows industrial use of extracted palladium without isotope separation.[9]
Silver-109
109Ag | 111Ag |
Cadmium-111 to 116
111Cd | 112Cd | 113Cd | 114Cd | 115Cd | 116Cd |
Indium-115
115In |
Tin-117 to 126
117Sn | 118Sn | 119Sn | 120Sn | 121Sn | 122Sn | 123Sn | 124Sn | 125Sn | 126Sn |
Antimony-121, 123, 124, 125
| 123Sb | 125Sb |
Tellurium-125 to 132
125Te | 126Te | 127Te | 128Te | 129Te | 130Te | 131Te | 132Te |
Tellurium-128 and -130 are essentially stable. They only decay by double beta decay, with half lives >1020 years. They constitute the major fraction of natural occurring tellurium at 32 and 34% respectively. Tellurium-132 and its daughter 132I are important in the first few days after a criticality. It was responsible for a large fraction of the dose inflicted on workers at Chernobyl in the first week.
The isobar forming 132Te/132I is: Tin-132 (half-life 40 s) decaying to antimony-132 (half-life 2.8 minutes) decaying to tellurium-132 (half-life 3.2 days) decaying to iodine-132 (half-life 2.3 hours) which decays to stable xenon-132.
The creation of tellurium-126 is delayed by the long half-life (230 k years) of tin-126.
Iodine-127, 129, 131
| 127I | 129I | 131I |
131I, with a half-life of 8 days, is a hazard from nuclear fallout because iodine concentrates in the thyroid gland. See also Radiation effects from Fukushima Daiichi nuclear disaster#Iodine-131 and Downwinders#Nevada.
In common with 89Sr, 131I is used for the treatment of cancer. A small dose of 131I can be used in a thyroid function test while a large dose can be used to destroy the thyroid cancer. This treatment will also normally seek out and destroy any secondary tumor which arose from a thyroid cancer. Much of the energy from the beta emission from the 131I will be absorbed in the thyroid, while the gamma rays are likely to be able to escape from the thyroid to irradiate other parts of the body.
Large amounts of 131I was released during an experiment named the Green Run[10] in which fuel which had only been allowed to cool for a short time after irradiation was reprocessed in a plant which had no iodine scrubber in operation.
129I, with a half-life almost a billion times as long, is a long-lived fission product.
127I is stable, the only one of the isotopes of iodine that is nonradioactive. It makes up only about 1⁄6 of the iodine in spent fuel, with I-129 about 5⁄6.
Xenon-131 to 136
131Xe | 132Xe | 133Xe | 134Xe | 135Xe | 136Xe |
In reactor fuel, the fission product xenon tends to migrate to form bubbles in the fuel. As caesium 133, 135, and 137 are formed by the beta particle decay of the corresponding xenon isotopes, this causes the caesium to become physically separated from the bulk of the uranium oxide fuel.
Because 135Xe is a potent nuclear poison with a large cross section for neutron absorption, the buildup of 135Xe in the fuel inside a power reactor can lower the reactivity greatly. If a power reactor is shut down or left running at a low power level, then large amounts of 135Xe can build up through decay of 135I. When the reactor is restarted or the low power level is increased significantly, 135Xe will be quickly consumed through neutron capture reactions and the reactivity of the core will increase. Under some circumstances, control systems may not be able to respond quickly enough to manage an abrupt reactivity increase as the built-up 135Xe burns off. It is thought that xenon poisoning was one of the factors which led to the power surge which damaged the Chernobyl reactor core.
Caesium-133, 134, 135, 137
| 133Cs | 134Cs | 135Cs | 137Cs |
Caesium-137 with a half-life of 30 years is the main medium-lived fission product, along with Sr-90. Cs-137 is the primary source of penetrating gamma radiation from spent fuel until 300 years or more after discharge. It is the most significant radioisotope left in the area around Chernobyl.[11]
Caesium-134 is found in spent nuclear fuel but is not produced by nuclear weapon explosions, as it is only formed by neutron capture on stable Cs-133, which is only produced by beta decay of Xe-133 with a half-life of 3 days. Cs-134 has a half-life of 2 years and may be a major source of gamma radiation in the first few years after discharge.
Caesium-135 is a long-lived fission product with much weaker radioactivity. Neutron capture inside the reactor diverts much of the xenon-135 that would otherwise decay to Cs-135.
Barium-138, 139, 140
| 138Ba | 139Ba | 140Ba |
A lot of barium is formed by the fission process, a short lived barium isotope was confused with radium by some early workers. They were bombarding uranium with neutrons in an attempt to form a new element. But instead they caused fission which generated a large amount of radioactivity in the target. Because the chemistry of barium and radium the two elements could be coseparated by for instance a precipitation with sulfate anions. Because of this similarity of their chemistry the early workers thought that the very radioactive fraction which was separated into the "radium" fraction contained a new isotope of radium. Some of this early work was done by Otto Hahn and Fritz Strassmann.
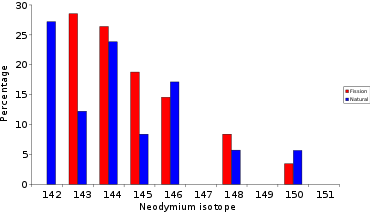
Lanthanides (lanthanum-139, cerium-140 to 144, neodymium-142 to 146, 148, 150, promethium-147, and samarium-149, 151, 152, 154)
| 139La | 140La | ||||||||||||||||||||||
| 140Ce | 141Ce | 142Ce | 143Ce | 144Ce | |||||||||||||||||||
| 141Pr | 143Pr | ||||||||||||||||||||||
| 143Nd | 144Nd | 145Nd | 146Nd | 147Nd | 148Nd | 149Nd | 150Nd | ||||||||||||||||
| 147Pm | 149Pm | 151Pm | |||||||||||||||||||||
| 147Sm | 149Sm | 151Sm | 152Sm | 153Sm | 154Sm | ||||||||||||||||||
| 153Eu | 154Eu | 155Eu | 156Eu | ||||||||||||||||||||
| 155Gd | 156Gd | 157Gd | 158Gd | 159Gd | 160Gd | ||||||||||||||||||
| 159Tb | 161Tb | ||||||||||||||||||||||
| 161Dy |
A great deal of the lighter lanthanides (lanthanum, cerium, neodymium, and samarium) are formed as fission products. In Africa, at Oklo where the natural nuclear fission reactor operated over a billion years ago, the isotopic mixture of neodymium is not the same as 'normal' neodymium, it has an isotope pattern very similar to the neodymium formed by fission.
In the aftermath of criticality accidents, the level of 140La is often used to determine the fission yield (in terms of the number of nuclei which underwent fission).
Samarium-149 is the second most important neutron poison in nuclear reactor physics. Samarium-151, produced at lower yields, is the third most abundant medium-lived fission product but emits only weak beta radiation. Both have high neutron absorption cross sections, so that much of them produced in a reactor are later destroyed there by neutron absorption.
External links

- Periodic Table with isotope decay chain displays. Click on element, then isotope mass number to see decay chain (link to uranium 235).
References
- http://www-pub.iaea.org/MTCD/publications/PDF/Pub886_web/Chernobylmap2.html
- https://www.tandfonline.com/doi/abs/10.1080/19443994.2013.848655
- https://patents.google.com/patent/US2945740
- http://www.nea.fr/html/pt/docs/iem/jeju02/session2/SessionII-14.pdf
- "Archived copy". Archived from the original on December 20, 2005. Retrieved March 12, 2006.CS1 maint: archived copy as title (link)
- Potential Applications of Fission Platinoids in Industry, Zdenek Kolarik, Platinum Metals Review, 2005, 49, April (2).
- http://www.orau.org/ptp/collection/hpposters/goldjewelry.htm
- http://www.iaea.org/Publications/Magazines/Bulletin/Bull413/article9.pdf
- Fang, Shengqiang; Fu, Lian; Pang, Changang (February 1996). "Recovery of palladium from basic reprocessing waste of spent nuclear fuel". Journal of Radioanalytical and Nuclear Chemistry. 203 (1): 143–149. doi:10.1007/BF02060389.
- "Archived copy". Archived from the original on May 8, 2006. Retrieved March 12, 2006.CS1 maint: archived copy as title (link)
- IAEA map
