Ferroptosis
Ferroptosis is a type of programmed cell death dependent on iron and characterized by the accumulation of lipid peroxides, and is genetically and biochemically distinct from other forms of regulated cell death such as apoptosis.[1] Ferroptosis is initiated by the failure of the glutathione-dependent antioxidant defenses, resulting in unchecked lipid peroxidation and eventual cell death.[2] Lipophilic antioxidants[3] and iron chelators can prevent ferroptotic cell death. Although the connection between iron and lipid peroxidation has been appreciated for years, [4] it was not until 2012 that Brent Stockwell and Scott Dixon coined the term ferroptosis and described several of its key features.[5]
Researchers have identified roles in which ferroptosis can contribute to the medical field such as in aiding in cancer treatments where this form of cell death can be induced in the human body.[6] Ferroptosis activation plays a regulatory role on growth of tumor cells in the human body. However, the positive effects of ferroptosis could be potentially neutralized by its disruption of metabolic pathways and disruption of homeostasis in the human body.[7] Since ferroptosis is a form of regulated cell death[8], some of the molecules that regulate ferroptosis are involved in metabolic pathways that regulate cysteine exploitation, glutathione state, nicotinamide adenine dinucleotide phosphate function, lipid peroxidation and iron homeostasis.[7]
Mechanism of ferroptosis
The hallmark feature of ferroptosis is the iron-dependent accumulation of oxidatively damaged phospholipids (i.e. lipid peroxides). This occurs when free radical molecules take electrons from a lipid molecule, promoting their oxidation by oxygen. The primary cellular mechanism of protection against ferroptosis is mediated by GPX4, a glutathione-dependent peroxidase that converts lipid peroxides into non-toxic lipid alcohols.[1] Recently, a second parallel protective pathway was independently discovered by two labs that involves the oxidoreductase FSP1/AIFM2.[9][10] Their findings indicate that FSP1/AIFM2 enzymatically reduces non-mitochondrial Coenzyme Q10, thereby generating a potent lipophilic antioxidant to suppresses the propagation of lipid peroxides.[9][10]
Small molecules such as erastin, sulfasalazine, sorafenib, altretamine, RSL-3, ML-162 and ML-210 are known inhibitors of this tumor cell growth and induce ferroptosis. They do not trigger a response of change in apoptosis and therefore have no chromatin margination or cleave poly ADP-ribose polymerase (PARP). Instead, the phenotype of the mitochondria is changed using primarily erastin or RSL3. Iron is also a necessity for these activators. They therefore can be inhibited by iron chelators. Ultimately, cell death induced by erastin and RSL-3 in the phenotype that makes up ferroptosis. Ferroptosis can also be induced by blocking the enzyme GPX4. In some cells, FSP1 compensates for loss of GPX4 activity and both GPX4 and FSP1 must be simultaneous inhibited to induce ferroptosis. Triggering ferroptosis is also caused by inhibiting GSH, which is necessary for the function of GPX4, and ultimately inducing a ferroptotic response in a cell.[2]
Comparison to apoptosis in the nervous system
Another form of cell death that occurs in the nervous system is apoptosis. Named after the Greek word meaning “to fall away from”, apoptosis results in cell breakage into small, apoptotic bodies taken up through phagocytosis.[11] This process occurs continuously within mammalian nervous system processes that begin at fetal development and continue through adult life. Apoptotic death is crucial for the correct population size of neuronal and glial cells. Similarly to ferroptosis, deficiencies in apoptotic processes can result in many health complications, including neurodegeneration.
Within the study of neuronal apoptosis, most research has been conducted on the neurons of the superior cervical ganglion.[12] In order for these neurons to survive and innervate their target tissues, they must have nerve growth factor (NGF).[12] Normally, NGF binds to a tyrosine kinase receptor, TrkA, which activates phosphatidylinositol 3-kinase-Akt (PI3K-Akt) and extracellular signal-regulated kinase (Raf-MEK-ERK) signaling pathways. This occurs during normal development which promotes neuronal growth in the sympathetic nervous system.[12]
During embryonic development, the absence of NGF activates apoptosis by decreasing the activity of the signaling pathways normally activated by NGF.[12] this pathway of apoptosis has also been named the intrinsic pathway, as it is activated by intrinsic factors over external factors. Without NGF, the neurons of the sympathetic nervous system begin to atrophy, glucose uptake rates fall and the rates of protein synthesis and gene expression slow.[12] Apoptotic death from NGF withdrawal also requires caspase activity.[12] Upon NGF withdrawal, caspase-3 activation occurs through an in-vitro pathway beginning with the release of Cytochrome c from the mitochondria.[12] In a surviving sympathetic neuron, the overexpression of anti-apoptotic B-cell CLL/lymphoma 2 (Bcl-2) proteins prevents NGF withdrawal-induced death. However, overexpression of a separate, pro-apoptotic Bcl-2 gene, Bax, stimulates the release of Cytochrome c2. Cytochrome c promotes the activation of caspase-9 through the formation of the apoptosome. Once caspase-9 is activated, it can cleave and activate caspase-3 resulting in cell death. Notably, apoptosis does not release intracellular fluid as neurons that are degraded though ferroptosis do. During ferroptosis, neurons release lipid metabolites from inside the cell body. This is a key difference between ferroptosis and apoptosis.
Ferroptosis in neurons

Neural connections are constantly changing within the nervous system. Synaptic connections that are used more often are kept intact and promoted, while synaptic connections that are rarely used are subject to degradation. Elevated levels of synaptic connection loss and degradation of neurons are linked to neurodegenerative diseases.[13] More recently, ferroptosis has been linked to diverse brain diseases.[14] Two new studies show that ferroptosis contributes to neuronal death after intracerebral hemorrhage.[15][16] Neurons that are degraded through ferroptosis release lipid metabolites from inside the cell body. The lipid metabolites are harmful to surrounding neurons, causing inflammation in the brain. Inflammation is a pathological feature of Alzheimer’s disease and intracerebral hemorrhage.
In a study performed using mice it was found that absence of a certain enzyme, glutathione peroxidase 4 (Gpx4), enhanced activity of ferroptosis. Foods high in Vitamin E promote Gpx4 activity, consequently inhibiting ferroptosis and preventing inflammation in brain regions. In the experimental group of mice that were manipulated to have decreased Gpx4 levels, mice were observed to have cognitive impairment and neurodegeneration of hippocampal neurons, again linking ferroptosis to neurodegenerative diseases.
Similarly, presence of transcription factors, specifically ATF-4, can determine how readily a neuron can undergo cell death. The presence of ATF-4 promotes resistance in cells against ferroptosis. However, this resistance can cause other diseases, such as cancer, to progress and become malignant. While ATF-4 provides resistance ferroptosis, an abundance of ATF-4 causes neurodegeneration.
Role of ferroptosis in cancer treatment
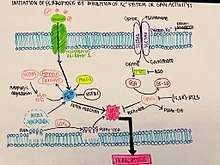
Ferroptosis is a more controlled form of programmed cell death. With small molecules inducing ferroptosis, it inhibits tumor growth and increases drug resistance. It is still unknown the exact mechanism in which ferroptosis inhibits tumor growth, but based on early experiments, it could be used to treat tumor cells and ultimately cancer. Based on the figure shown, initiating ferroptosis can be done by eliminating GPX4 activity through Xc- (erastin mediated). The inhibition or degradation of GPX4 by erastin or RSL3 has been noticed to be the primary mechanism that will trigger ferroptosis. Since ferroptosis is an iron dependent mechanism, an accumulation of lipid ROS kills the cells that have begun to undergo ferroptosis and eliminates them completely.
Ferroptosis can be used to treat several different types of cancer, each seemingly different forms of the disease. This method of cell death has been tested either in mice, or is in the early stages of research and has not yet been fully tested. This includes cancer types such as:
- Breast
- Acute myeloid leukemia
- Pancreatic ductal adenocarcinoma
- Ovarian
- B cell lymphoma
- Renal cell carcinomas
- Lung
- Glioblastoma
These forms of cancer have been hypothesized to be highly sensitive to ferroptosis, or react to erastin or Xc- in a way that minimizes the tumor cells or responds to this treatment with a result of cell death. An upregulation of iron levels has also been seen to induce ferroptosis in certain types of cancer, such as breast cancer.[6] Breast cancer cells have exhibited vulnerability to ferroptosis via a combination of siramesine and lapatinib. These cells also exhibited an autophagic cycle independent of ferroptotic activity, indicating that the two different forms of cell death could be controlled to activate at specific times following treatment.[17]
References
- Yang WS, Stockwell BR (March 2016). "Ferroptosis: Death by Lipid Peroxidation". Trends in Cell Biology. 26 (3): 165–176. doi:10.1016/j.tcb.2015.10.014. PMC 4764384. PMID 26653790.
- Cao JY, Dixon SJ (June 2016). "Mechanisms of ferroptosis". Cellular and Molecular Life Sciences. 73 (11–12): 2195–209. doi:10.1007/s00018-016-2194-1. PMC 4887533. PMID 27048822.
- Zilka O, Shah R, Li B, Friedmann Angeli JP, Griesser M, Conrad M, Pratt DA (March 2017). "On the Mechanism of Cytoprotection by Ferrostatin-1 and Liproxstatin-1 and the Role of Lipid Peroxidation in Ferroptotic Cell Death". ACS Central Science. 3 (3): 232–243. doi:10.1021/acscentsci.7b00028. PMC 5364454. PMID 28386601.
- Gutteridge, J.M. (1984). "Lipid peroxidation initiated by superoxide-dependent hydroxyl radicals using complexed iron and hydrogen peroxide". FEBS Letters. 172 (2): 245–249. doi:10.1016/0014-5793(84)81134-5. PMID 6086389.
- Dixon, Scott J.; Lemberg, Kathryn M.; Lamprecht, Michael R.; Skouta, Rachid; Zaitsev, Eleina M.; Gleason, Caroline E.; Patel, Darpan N.; Bauer, Andras J.; Cantley, Alexandra M.; Yang, Wan Seok; Morrison, Barclay (May 2012). "Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death". Cell. 149 (5): 1060–1072. doi:10.1016/j.cell.2012.03.042. PMC 3367386. PMID 22632970.
- Lu B, Chen XB, Ying MD, He QJ, Cao J, Yang B (12 January 2018). "The Role of Ferroptosis in Cancer Development and Treatment Response". Frontiers in Pharmacology. 8: 992. doi:10.3389/fphar.2017.00992. PMC 5770584. PMID 29375387.
- Hao S, Liang B, Huang Q, Dong S, Wu Z, He W, Shi M (April 2018). "Metabolic networks in ferroptosis". Oncology Letters. 15 (4): 5405–5411. doi:10.3892/ol.2018.8066. PMC 5844144. PMID 29556292.
- Nirmala GJ and Lopus M (2020) Cell death mechanisms in eukaryotes. Cell Biol Toxicol, 36, 145–164. doi: /10.1007/s10565-019-09496-2. PMID: 31820165
- Bersuker K, Hendricks JM, Li Z, Magtanong L, Ford B, Tang PH, et al. (November 2019). "The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis". Nature. 575 (7784): 688–692. Bibcode:2019Natur.575..688B. doi:10.1038/s41586-019-1705-2. PMC 6883167. PMID 31634900.
- Doll S, Freitas FP, Shah R, Aldrovandi M, da Silva MC, Ingold I, et al. (November 2019). "FSP1 is a glutathione-independent ferroptosis suppressor". Nature. 575 (7784): 693–698. Bibcode:2019Natur.575..693D. doi:10.1038/s41586-019-1707-0. PMID 31634899.
- Reed JC (November 2000). "Mechanisms of apoptosis". The American Journal of Pathology. 157 (5): 1415–30. doi:10.1016/S0002-9440(10)64779-7. PMC 1885741. PMID 11073801.
- Kristiansen M, Ham J (July 2014). "Programmed cell death during neuronal development: the sympathetic neuron model". Cell Death and Differentiation. 21 (7): 1025–35. doi:10.1038/cdd.2014.47. PMC 4207485. PMID 24769728.
- Hambright WS, Fonseca RS, Chen L, Na R, Ran Q (August 2017). "Ablation of ferroptosis regulator glutathione peroxidase 4 in forebrain neurons promotes cognitive impairment and neurodegeneration". Redox Biology. 12: 8–17. doi:10.1016/j.redox.2017.01.021. PMC 5312549. PMID 28212525.
- Weiland A, Wang Y, Wu W, Lan X, Han X, Li Q, Wang J (July 2019). "Ferroptosis and Its Role in Diverse Brain Diseases". Molecular Neurobiology. 56 (7): 4880–4893. doi:10.1007/s12035-018-1403-3. PMC 6506411. PMID 30406908.
- Li Q, Han X, Lan X, Gao Y, Wan J, Durham F, et al. (April 2017). "Inhibition of neuronal ferroptosis protects hemorrhagic brain". JCI Insight. 2 (7): e90777. doi:10.1172/jci.insight.90777. PMC 5374066. PMID 28405617.
- Li Q, Weiland A, Chen X, Lan X, Han X, Durham F, et al. (July 2018). "Ultrastructural Characteristics of Neuronal Death and White Matter Injury in Mouse Brain Tissues After Intracerebral Hemorrhage: Coexistence of Ferroptosis, Autophagy, and Necrosis". Frontiers in Neurology. 9: 581. doi:10.3389/fneur.2018.00581. PMC 6056664. PMID 30065697.
- Ma S, Dielschneider RF, Henson ES, Xiao W, Choquette TR, Blankstein AR, et al. (2017). "Ferroptosis and autophagy induced cell death occur independently after siramesine and lapatinib treatment in breast cancer cells". PLOS ONE. 12 (8): e0182921. Bibcode:2017PLoSO..1282921M. doi:10.1371/journal.pone.0182921. PMC 5565111. PMID 28827805.