Ethoxyquin
Ethoxyquin (EMQ) is a quinoline-based antioxidant used as a food preservative in certain countries and originally to control scald on pears after harvest (under commercial names such as "Stop-Scald").[2] It is used as a preservative in some pet foods to slow the development of rancidity of fats. Ethoxyquin is also used in some spices to prevent color loss due to oxidation of the natural carotenoid pigments.[3]
 | |
| Names | |
|---|---|
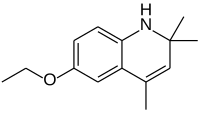
| IUPAC name
6-Ethoxy-2,2,4-trimethyl-1,2-dihydroquinoline | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.001.887 |
| E number | E324 (antioxidants, ...) |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C14H19NO | |
| Molar mass | 217.312 g·mol−1 |
| Melting point | < 25 °C (77 °F; 298 K) |
| Boiling point | 123–125 °C (253–257 °F; 396–398 K) at 2 mmHg |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Regulation
Ethoxyquin was developed by Monsanto in the 1950s. Ethoxyquin was initially registered as a pesticide in 1965 as an antioxidant used as a deterrent of scald in pears through post-harvest indoor application via a drench and/or impregnated wrap.[3]
As an antioxidant to control the browning of pears, ethoxyquin is approved in the United States[3] and in the European Union.[2]
In the United States, it is approved for use as an animal feed additive[4] and is limited as a food additive to use only in the spices chili powder, paprika, and ground chili.[5] Ethoxyquin is not permitted for use as food additive in Australia nor within the European Union.[6]
Ethoxyquin is allowed in the fishing industry in Norway and France as a feed stabilizer, so is commonly used in food pellets fed to farmed salmon.[7]
Ethoxyquin is used in pellets fed to chickens on chicken farms.[8]
In 2017 the EU suspended authorization for use as a feed additive, with various dates between 2017 and 2019 for final allowance of sale of goods so that alternatives may be phased in.[9]
Safety
Some speculation exists that ethoxyquin in pet foods might be responsible for multiple health problems. To date, the U.S. Food and Drug Administration has only found a verifiable connection between ethoxyquin and buildup of protoporphyrin IX in the liver, as well as elevations in liver-related enzymes in some animals, but no health consequences from these effects are known.[10] In 1997, the Center for Veterinary Medicine asked pet food manufacturers to voluntarily limit ethoxyquin levels to 75 ppm until further evidence is reported.[10] However, most pet foods that contain ethoxyquin have never exceeded this amount.[10] In 2017, reports from the Swiss Department for regional affairs laboratory, service of consummation and veterinary affairs showed that farmed salmon often exceeded the set limits for ethoxyquin contamination by several orders of magnitude and that health effects of the chemical on the human body were not studied in sufficient detail[11]. In 2013, researchers at the Department of General Genetics, Molecular Biology and Plant Biotechnology, Faculty of Biology and Environmental Protection, University of Łódź, Poland, summarized the health effects of animals and humans exposed to varying levels of ethoxyquin observed in scientific studies. The summary includes: loss of weight, changes in liver, kidney, alimentary duct, urinary bladder and mitochondria, anemia, lethargy, discolored urine, skin, or fur, increase in mortality, detrimental effect on immunity, condition factor of final body weight in relation to body length of fish and inducement of allergies (contact exposure).[12]
2015 EFSA review
A 2015 review by the European Food Safety Authority indicated that data to assess the safety of ethoxyquin as a feed additive for target animals, or its safety for consumers and the environment are lacking.[13] The agency found one of its metabolites, ethoxyquin quinone imine, to be possibly genotoxic, and p-phenetidine, an impurity that could be present from the manufacturing process, to be possibly mutagenic.[2] In response, feed manufacturers have taken steps to significantly reduce the amount of p-phenetidine in their products.[14]
References
- Merck Index, 11th Edition, 3710
- EFSA Ethoxiquin
- "R.E.D. FACTS Ethoxyquin" (PDF). United States Environmental Protection Agency. November 2004. EPA-738-F-04-006. Cite journal requires
|journal=(help) - 21 C.F.R. 573.380
- 21 C.F.R. 172.140
- "COMMISSION IMPLEMENTING REGULATION (EU) 2017/962 of 7 June 2017 suspending the authorisation of ethoxyquin as a feed additive for all animal species and categories". European Union. Cite journal requires
|journal=(help) - Lundebye AK, Hove H, Måge A, Bohne VJ, Hamre K (2010). "Levels of synthetic antioxidants (ethoxyquin, butylated hydroxytoluene and butylated hydroxyanisole) in fish feed and commercially farmed fish". Food Addit Contam Part a Chem Anal Control Expo Risk Assess. 27 (12): 1652–7. doi:10.1080/19440049.2010.508195. PMID 20931417.
- Rubel DM, Freeman S (May 1998). "Allergic contact dermatitis to ethoxyquin in a farmer handling chicken feeds". Australasian Journal of Dermatology. 39 (2): 89–91. doi:10.1111/j.1440-0960.1998.tb01255.x. PMID 9611377.
- https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32017R0962
- "Pet Food Labels - General". U.S. Food and Drug Administration. October 13, 2017. Retrieved August 16, 2019.
- Farmed Norwegian Salmon World's Most Toxic Food, retrieved 2019-11-20
- Alina Błaszczyk, Aleksandra Augustyniak, Janusz Skolimowski (15 January 2013). "Ethoxyquin: An Antioxidant Used in Animal Feed". International Journal of Food Science. 2013: 585931. doi:10.1155/2013/585931. PMC 4745505. PMID 26904606.CS1 maint: uses authors parameter (link)
- "Ethoxyquin: EFSA safety assessment inconclusive". EFSA. November 15, 2015. Retrieved December 14, 2016.
- "What is the future of ethoxyquin in the EU?". Cite journal requires
|journal=(help)