Ether lipid
In an organic chemistry general sense, an ether lipid implies an ether bridge between an alkyl group (a lipid) and an unspecified alkyl or aryl group, not necessarily glycerol. If glycerol is involved, the compound is called a glyceryl ether, which may take the form of an alkylglycerol, an alkyl acyl glycerol, or in combination with a phosphatide group, a phospholipid.
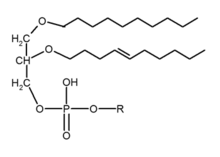

In a biochemical sense, an ether lipid usually implies glycerophospholipids of various type, also called phospholipids, in which the sn-1 position of the glycerol backbone has a lipid attached by an ether bond and a lipid attached to the sn-2 position via an acyl group. This is in contrast to the more common glycerophospholipids, 1,2-diacyl-sn-glycerol (DAG), in which the glycerol backbone sn-1 and sn-2 positions have acyl chains attached by ester bonds.[1][2] Ether lipid may also refer to alkylglycerols, such as chimyl (16:0), batyl (18:0), and selachyl (18:1 n-9) alcohols, with an ether-bound lipid on position sn-1, and the other two positions on the glycerol backbone unoccupied.[3]
Types
There are two types of ether lipids, plasmanyl- and plasmenyl-phospholipids. Plasmanyl-phospholipids have an ether bond in position sn-1 to an alkyl group. Plasmenyl-phospholipids have an ether bond in position sn-1 to an alkenyl group, 1-0-alk-1’-enyl-2-acyl-sn-glycerol (AAG).[2] The latter type is called plasmalogens.[4]
Platelet-activating factor (PAF) is an ether lipid which has an acetyl group instead of an acyl chain at the second position (SN-2).
Biosynthesis
The formation of the ether bond in mammals requires two enzymes, dihydroxyacetonephosphate acyltransferase (DHAPAT) and alkyldihydroxyacetonephosphate synthase (ADAPS), that reside in the peroxisome.[5] Accordingly, peroxisomal defects often lead to impairment of ether-lipid production.
Monoalkylglycerol ethers (MAGEs) are also generated from 2-acetyl MAGEs (precursors of PAF) by KIAA1363.
Functions
Structural
Plasmalogens as well as some 1-O-alkyl lipids are ubiquitous and sometimes major parts of the cell membranes in mammals and anaerobic bacteria.[6] In archaea, ether lipids are the major polar lipids in the cell envelope and their abundance is one of the major characteristics that separate this group of prokaryotes from the bacteria. In these cells, diphytanylglycerolipids or bipolar macrocyclic tetraethers can form covalently linked 'bilayers'.[7]
Second messenger
Differences between the catabolism of ether glycerophospholipids by specific phospholipases enzymes might be involved in the generation of lipid second messenger systems such as prostaglandins and arachidonic acid that are important in signal transduction.[8] Ether lipids can also act directly in cell signaling, as the platelet-activating factor is an ether lipid signaling molecule that is involved in leukocyte function in the mammalian immune system.[9]
Antioxidant
Another possible function of the plasmalogen ether lipids is as antioxidants, as protective effects against oxidative stress have been demonstrated in cell culture and these lipids might therefore play a role in serum lipoprotein metabolism.[10] This antioxidant activity comes from the enol ether double bond being targeted by a variety of reactive oxygen species.[11]
Synthetic ether lipid analogs
Synthetic ether lipid analogs have cytostatic and cytotoxic properties, probably by disrupting membrane structure and acting as inhibitors of enzymes within signal transmission pathways, such as protein kinase C and phospholipase C.
A toxic ether lipid analogue miltefosine has recently been introduced as an oral treatment for the tropical disease leishmaniasis, which is caused by leishmania, a protozoal parasite with a particularly high ether lipid content in its membranes.[12]
See also
References
- Dean JM, Lodhi IJ (February 2018). "Structural and functional roles of ether lipids". Protein & Cell. 9 (2): 196–206. doi:10.1007/s13238-017-0423-5. PMC 5818364. PMID 28523433.
- Ford DA, Gross RW (July 1990). "Differential metabolism of diradyl glycerol molecular subclasses and molecular species by rabbit brain diglyceride kinase". The Journal of Biological Chemistry. 265 (21): 12280–6. PMID 2165056. S2CID 1042240.
- Christie W. "Ether lipids - glyceryl ethers, plasmalogens, aldehydes, structure, biochemistry, composition and analysis". www.lipidhome.co.uk.
- Watson RR, De Meester F, eds. (2014). Omega 3 fatty acids in brain and neurological health. Elsevier Academic Press. doi:10.1016/C2012-0-06006-1. ISBN 978-0-12-410527-0.
- Hajra AK (1995). "Glycerolipid biosynthesis in peroxisomes (microbodies)". Progress in Lipid Research. 34 (4): 343–64. doi:10.1016/0163-7827(95)00013-5. PMID 8685243.
- Paltauf F (December 1994). "Ether lipids in biomembranes". Chemistry and Physics of Lipids. 74 (2): 101–39. doi:10.1016/0009-3084(94)90054-X. PMID 7859340.
- Koga Y, Morii H (November 2005). "Recent advances in structural research on ether lipids from archaea including comparative and physiological aspects". Bioscience, Biotechnology, and Biochemistry. 69 (11): 2019–34. doi:10.1271/bbb.69.2019. PMID 16306681.
- Spector AA, Yorek MA (September 1985). "Membrane lipid composition and cellular function". Journal of Lipid Research. 26 (9): 1015–35. PMID 3906008.
- Demopoulos CA, Pinckard RN, Hanahan DJ (October 1979). "Platelet-activating factor. Evidence for 1-O-alkyl-2-acetyl-sn-glyceryl-3-phosphorylcholine as the active component (a new class of lipid chemical mediators)". The Journal of Biological Chemistry. 254 (19): 9355–8. PMID 489536.
- Brosche T, Platt D (August 1998). "The biological significance of plasmalogens in defense against oxidative damage". Experimental Gerontology. 33 (5): 363–9. doi:10.1016/S0531-5565(98)00014-X. PMID 9762517.
- Engelmann B (February 2004). "Plasmalogens: targets for oxidants and major lipophilic antioxidants". Biochemical Society Transactions. 32 (Pt 1): 147–50. doi:10.1042/BST0320147. PMID 14748736.
- Lux H, Heise N, Klenner T, Hart D, Opperdoes FR (November 2000). "Ether--lipid (alkyl-phospholipid) metabolism and the mechanism of action of ether--lipid analogues in Leishmania". Molecular and Biochemical Parasitology. 111 (1): 1–14. doi:10.1016/S0166-6851(00)00278-4. PMID 11087912.
External links
- Ether+phospholipids at the US National Library of Medicine Medical Subject Headings (MeSH)