Ehrlich's reagent
Ehrlich's reagent or Ehrlich reagent is a reagent that contains p-dimethylaminobenzaldehyde (DMAB) and thus can act as an indicator to presumptively identify indoles and urobilinogen. Several Ehrlich tests use the reagent in a medical test; some are drug tests and others contribute to diagnosis of various diseases or adverse drug reactions. A very common Ehrlich test is a simple spot test to identify possible psychoactive compounds such as tryptamines (e.g. DMT) and ergoloids (e.g. LSD). The reagent will also give a positive result for opium, despite the opiates not containing the indole functional group, because of the presence of tryptophan in natural opium.[1] It is named after Nobel Prize winner Paul Ehrlich who used it to distinguish typhoid from simple diarrhoea.
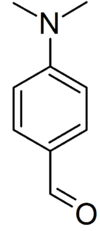
The reagent is prepared by dissolving 0.5[2]–2.0 g of p–dimethylaminobenzaldehyde (DMAB) in 50 mL of 95% ethanol and 50 mL of concentrated hydrochloric acid[3][4] and is best used when fresh. Other alcohols, such as 1-propanol, can also be used as well.[5]
The Ehrlich reagent is similar to a number of other indole tests:
- The van Urk reagent, which uses p-DMAB, sulfuric acid and an oxidant.[6]
- The Renz and Loew reagent, which uses p-dimethylaminocinnamaldehyde and may also be used for the detection of flavonoids.
- The "improved hallucinogen reagent", which uses a 1:1 solution of 5% DMAB in concentrated phosphoric acid (specific gravity 1.75) to methanol.[1][7]
- The Hofmann Reagent, which uses 0.125 g of p-DMAB, 0.2 mL of ferric chloride solution (25 g/mL) in a solution of 65% sulfuric acid.[8][9] This is sometimes referred to as p-DMAB-TS (Test Solution) and gives slightly different colours with different indoles.
The Ehrlich reagent works by binding to the C2 position of two indole moieties to form a resonance stabilised carbenium ion compound.[10]
See also
- Pill testing
- Other alkaloid spot tests:
- Froehde reagent
- Indole test
- Kovac's reagent, similar but uses isoamyl alcohol
- Liebermann reagent
- Marquis reagent
References
- de Faubert Maunder, MJ (1975). "Field and laboratory test for raw and prepared opium". Bulletin on narcotics. 27 (1): 71–6. PMID 1039285.
- Spratley, Trinette (2004). "Analytical Profiles for Five "Designer" Tryptamines" (PDF). Microgram Journal. 3 (1–2): 55. Retrieved 2013-10-09.
- O’Neal, Carol L; Crouch, Dennis J; Fatah, Alim A (April 2000). "Validation of twelve chemical spot tests for the detection of drugs of abuse". Forensic Science International. 109 (3): 189–201. doi:10.1016/S0379-0738(99)00235-2. PMID 10725655.
- "Color Test Reagents/Kits for Preliminary Identification of Drugs of Abuse" (PDF). Law Enforcement and Corrections Standards and Testing Program. July 2000. Retrieved 2011-07-24.
- "Ehrlich's Reagent Safety Data Sheet" (PDF). Labchem. 2 July 2014. Retrieved 11 January 2015.
- Ehmann, A. (1977). "The van URK-Salkowski reagent — a sensitive and specific chromogenic reagent for silica gel thin-layer chromatographic detection and identification of indole derivatives" (PDF). Journal of Chromatography A. 132 (2): 267–276. doi:10.1016/S0021-9673(00)89300-0.
- Maunder, M. J. de Faubert (August 1974). "A field test for hallucinogens: further improvements". Journal of Pharmacy and Pharmacology. 26 (8): 637–638. doi:10.1111/j.2042-7158.1974.tb10677.x.
- Sunshine, Irving (1969). Handbook of analytical toxicology. Chemical Rubber Co. p. 408.
p-DMAB-TS: To a cool soln of 65 ml H2S04 in 35 ml H20, add 125 mg para-dimethylaminobenzaldehyde, dissolve, add 1-2 drops of FeCI3-TS.
- "Basic Tests for Pharmaceutical Substances: 5. Reagents". WHO: Essential Medicines and Health Products Information Portal. Retrieved 2019-12-20.
- Kovar, Karl-Artur; Laudszun, Martina (February 1989). "Chemistry and Reaction Mechanisms of Rapid Tests for Drugs of Abuse and Precursors Chemicals" (PDF). UNODC. p. 15. Retrieved 3 January 2016.
External links