Dissolved organic carbon
Dissolved organic carbon (DOC) is the fraction of total organic carbon operationally defined as that which can pass through a filter size that typically ranges in size from 0.22 and 0.7 micrometers.[1] The fraction remaining on the filter is called particulate organic carbon (POC).[2]
DOC is abundant in marine and freshwater systems and is one of the greatest cycled reservoirs of organic matter on Earth, accounting for the same amount of carbon as the atmosphere and up to 20% of all organic carbon.[3] In general, organic carbon compounds are the result of decomposition processes from dead organic matter including plants and animals. DOC can originate from within or external to the body of water. DOC originating from within the body of water is known as autochthonous DOC and typically comes from aquatic plants or algae, while DOC originating external to the body of water is known as allochthonous DOC and typically comes from soils or terrestrial plants.[4] When water originates from land areas with a high proportion of organic soils, these components can drain into rivers and lakes as DOC.
The marine DOC pool is important in the functioning of marine ecosystems, because it is at the interface between the chemical and the biological worlds, it fuels marine food webs, and is a major component of the Earth’s carbon cycling.[5]
Measurement
The dissolved fraction of total organic carbon (TOC) is an operational classification. Many researchers use the term "dissolved" for compounds that pass through a 0.45 μm filter, but 0.22 μm filters have also been used to remove higher colloidal concentrations.
A practical definition of dissolved typically used in marine chemistry is all substances that pass through a GF/F filter, which has a nominal pore size of approximately 0.7 μm (Whatman glass microfiber filter, 0.6–0.8 μm particle retention[6]). The recommended procedure is the HTCO technique, which calls for filtration through pre-combusted glass fiber filters, typically the GF/F classification.[7]
Significance
DOC is a food supplement, supporting growth of microorganisms and plays an important role in the global carbon cycle through the microbial loop.[8] In some organisms (stages) that do not feed in the traditional sense, dissolved matter may be the only external food source.[9] Moreover, DOC is an indicator of organic loadings in streams, as well as supporting terrestrial processing (e.g., within soil, forests, and wetlands) of organic matter. Dissolved organic carbon has a high proportion of biodegradable dissolved organic carbon (BDOC) in first order streams compared to higher order streams. In the absence of extensive wetlands, bogs, or swamps, baseflow concentrations of DOC in undisturbed watersheds generally range from approximately 1 to 20 mg/L carbon.[10] Carbon concentrations considerably vary across ecosystems. For example, the Everglades may be near the top of the range and the middle of oceans may be near the bottom. Occasionally, high concentrations of organic carbon indicate anthropogenic influences, but most DOC originates naturally.[11]
The BDOC fraction consists of organic molecules that heterotrophic bacteria can use as a source of energy and carbon. [12] Some subset of DOC constitutes the precursors of disinfection byproducts for drinking water.[13] BDOC can contribute to undesirable biological regrowth within water distribution systems.[14]
Distribution
More precise measurement techniques developed in the late 1990s have allowed for a good understanding of how dissolved organic carbon is distributed in marine environments both vertically and across the surface.[15] It is now understood that dissolved organic carbon in the ocean spans a range from very labile to very refractory. The labile dissolved organic carbon is mainly produced by marine organisms and is consumed in the surface ocean, and consists of sugars, proteins, and other compounds that are easily used by marine bacteria.[16] The refractory dissolved organic carbon is evenly spread throughout the water column and consists of high molecular weight and structurally complex compounds that are difficult for marine organisms to use such as the lignin, pollen, or humic acids.[17] Therefore, the observed vertical distribution consists of high concentrations in the upper water column and low concentrations at depth.
In addition to vertical distributions, horizontal distributions have been modeled and sampled as well.[18] In the surface ocean at a depth of 30 meters, the higher dissolved organic carbon concentrations are found in the South Pacific Gyre, the South Atlantic Gyre, and the Indian Ocean. At a depth of 3,000 meters, highest concentrations are in the North Atlantic Deep Water where dissolved organic carbon from the high concentration surface ocean is removed to depth. While in the northern Indian Ocean high DOC is observed due to high fresh water flux and sediments. Since the time scales of horizontal motion along the ocean bottom are in the thousands of years, the refractory dissolved organic carbon is slowly consumed on its way from the North Atlantic and reaches a minimum in the North Pacific.
Ocean sources
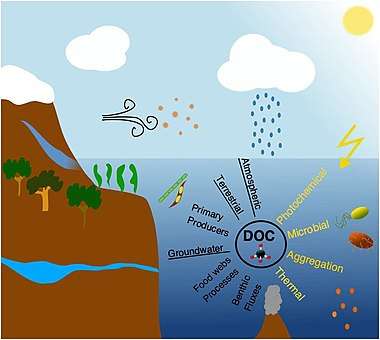
In marine systems DOC originates from either autochthonous or allochthonous sources. Autochthonous DOC is produced within the system, primarily by plankton organisms [19][20] and in coastal waters additionally by benthic microalgae, benthic fluxes, and macrophytes,[21] whereas allochthonous DOC is mainly of terrestrial origin supplemented by groundwater and atmospheric inputs.[22][23] In addition to soil derived humic material, terrestrial DOC also includes material leached from plants exported during rain events, emissions of plant materials to the atmosphere and deposition in aquatic environments (e.g., volatile organic carbon and pollens), and also thousands of synthetic human-made organic chemicals that can be measured in the ocean at “trace” concentrations.[24][25][5]
Phytoplankton produces DOC by extracellular release commonly accounting between 5 and 30% of their total primary production,[26] although this varies from species to species.[27] Nonetheless, this release of extracellular DOC is enhanced under high light and low nutrient levels, and thus should increase relatively from eutrophic to oligotrophic areas, probably as a mechanism for dissipating cellular energy.[28] Phytoplankton can also produce DOC by autolysis during physiological stress situations e.g., nutrient limitation.[29] Other studies have demonstrated DOC production in association with meso- and macro-zooplankton feeding on phytoplankton and bacteria.[30][5]
Zooplankton-mediated release of DOC occurs through sloppy feeding, excretion and defecation which can be important energy sources for microbes.[31][30] Such DOC production is largest during periods with high food concentration and dominance of large zooplankton species.[32][5]
Bacteria are often viewed as the main consumers of DOC, but they can also produce DOC during cell division and viral lysis.[33][34][35] The biochemical components of bacteria are largely the same as other organisms, but some compounds from the cell wall are unique and are used to trace bacterial derived DOC (e.g., peptidoglycan). These compounds are widely distributed in the ocean, suggesting that bacterial DOC production could be important in marine systems.[36] Viruses are the most abundant life forms in the oceans infecting all life forms including algae, bacteria and zooplankton.[37] After infection, the virus either enters a dormant (lysogenic) or productive (lytic) state.[38] The lytic cycle causes disruption of the cell(s) and release of DOC.[39][5]
Marine macrophytes (i.e., macroalgae and seagrass) are highly productive and extend over large areas in coastal waters but their production of DOC has not received much attention. Macrophytes release DOC during growth with a conservative estimate (excluding release from decaying tissues) suggesting that macroalgae release between 1-39% of their gross primary production,[40][41] while seagrasses release less than 5% as DOC of their gross primary production.[42] The released DOC has been shown to be rich in carbohydrates, with rates depending on temperature and light availability.[43][44] Globally the macrophyte communities have been suggested to produce ∼160 Tg C yr–1 of DOC, which is approximately half the annual global river DOC input (250 Tg C yr–1).[43][5]
Marine sediments represent the main sites of OM degradation and burial in the ocean, hosting microbes in densities up to 1000 times higher than found in the water column.[45] The DOC concentrations in sediments are often an order of magnitude higher than in the overlying water column.[46] This concentration difference results in a continued diffusive flux and suggests that sediments are a major DOC source releasing 350 Tg C yr–1, which is comparable to the input of DOC from rivers.[47] This estimate is based on calculated diffusive fluxes and does not include resuspension events which also releases DOC [48] and therefore the estimate could be conservative. Also, some studies have shown that geothermal systems and petroleum seepage contribute with pre-aged DOC to the deep ocean basins,[49][50] but consistent global estimates of the overall input are currently lacking. Globally, groundwaters account for an unknown part of the freshwater DOC flux to the oceans.[51] The DOC in groundwater is a mixture of terrestrial, infiltrated marine, and in situ microbially produced material.[52] This flux of DOC to coastal waters could be important, as concentrations in groundwater are generally higher than in coastal seawater,[53] but reliable global estimates are also currently lacking.[5]
Ocean sinks
The main processes that remove DOC from the ocean water column are: (1) Thermal degradation in e.g., submarine hydrothermal systems;[54] (2) bubble coagulation and abiotic flocculation into microparticles [55] or sorption to particles;[56] (3) abiotic degradation via photochemical reactions;[57][58] and (4) biotic degradation by heterotrophic marine prokaryotes.[59] It has been suggested that the combined effects of photochemical and microbial degradation represent the major sinks of DOC.[60][5]
Thermal degradation of DOC has been found at high-temperature hydrothermal ridge-flanks, where outflow DOC concentrations are lower than in the inflow. While the global impact of these processes has not been investigated, current data suggest it is a minor DOC sink.[61] Abiotic DOC flocculation is often observed during rapid (minutes) shifts in salinity when fresh and marine waters mix.[62] Flocculation changes the DOC chemical composition, by removing humic compounds and reducing molecular size, transforming DOC to particulate organic flocs which can sediment and/or be consumed by grazers and filter feeders, but it also stimulates the bacterial degradation of the flocculated DOC.[63] The impacts of flocculation on the removal of DOC from coastal waters are highly variable with some studies suggesting it can remove up to 30% of the DOC pool,[64][65] while others find much lower values (3–6%;[66]). Such differences could be explained by seasonal and system differences in the DOC chemical composition, pH, metallic cation concentration, microbial reactivity, and ionic strength.[62][67][5]
Interaction with metals
DOC is also extremely important in the transport of metals in aquatic systems. Metals form complexes with DOC, enhancing metal solubility while also reducing metal bioavailability.
See also
References
- "Organic Carbon". Bio-geochemical Methods. Retrieved 2018-11-27.
- Kenny, Jonathan E.; Bida, Morgan; Pagano, Todd (October 2014). "Trends in Levels of Allochthonous Dissolved Organic Carbon in Natural Water: A Review of Potential Mechanisms under a Changing Climate". Water. 6 (10): 2862–2897. doi:10.3390/w6102862.
- Hedges, John I. (3 December 1991). "Global biogeochemical cycles: progress and problems" (PDF). Marine Chemistry. 39 (1–3): 67–93. doi:10.1016/0304-4203(92)90096-s.
- Kritzberg, Emma S.; Cole, Jonathan J.; Pace, Michael L.; Granéli, Wilhelm; Bade, Darren L. (March 2004). "Autochthonous versus allochthonous carbon sources of bacteria: Results from whole-lake 13C addition experiments" (PDF). Limnology and Oceanography. 49 (2): 588–596. doi:10.4319/lo.2004.49.2.0588. ISSN 0024-3590.
- Lønborg, C., Carreira, C., Jickells, T. and Álvarez-Salgado, X.A. (2020) "Impacts of global change on ocean dissolved organic carbon (DOC) cycling". Frontiers in Marine Science, 7: 466. doi:10.3389/fmars.2020.00466.

- "Whatman® glass microfiber filters, Grade GF/F". Merck.
- Knap, A. Michaels; A. Close; A. Ducklow; H. Dickson, A. (1994). Protocols for the Joint Global Ocean Flux studies (JGOFS) core measurements. JGOFS.
- Kirchman, David L.; Suzuki, Yoshimi; Garside, Christopher; Ducklow, Hugh W. (15 August 1991). "High turnover rates of dissolved organic carbon during a spring phytoplankton bloom". Nature. 352 (6336): 612–614. Bibcode:1991Natur.352..612K. doi:10.1038/352612a0.
- Jaeckle, W.B.; Manahan, D.T. (1989). "Feeding by a "nonfeeding" larva: uptake of dissolved amino acids from seawater by lecithotrophic larvae of the gastropod Haliotis rufescens". Marine Biology. 103: 87–94. doi:10.1007/BF00391067.
- Cheremisinoff, Nicholas; Davletshin, Anton (2015). "Hydraulic Fracturing Operations: Handbook of Environmental Management Practices". Environmental Management.
- Elser, Stephen (2014). "Brown Water: The Ecological and Economic Implications of Increased Dissolved Organic Carbon in Lakes". Cite journal requires
|journal=(help) - Wu, Qing; Zhao, Xin-Hua; Wang, Xiao-Dan (2008). "Relationship Between Heterotrophic Bacteria and Some Physical and Chemical Parameters in a Northern City's Drinking Water Distribution Networks of China". doi:10.1109/ICBBE.2008.336. Cite journal requires
|journal=(help) - "Dissolved Organic Carbon (DOC)".
- Narayana, P.S.; Varalakshmi, D; Pullaiah, T; Sambasiva Rao, K.R.S. (2018). "Research Methodology in Zoology": 225. Cite journal requires
|journal=(help) - Sharp, Jonathan H. (6 August 1996). "Marine dissolved organic carbon: Are the older values correct?". Marine Chemistry. 56 (3–4): 265–277. doi:10.1016/S0304-4203(96)00075-8.
- Sondergaard, Morten; Mathias Middelboe (9 March 1995). "A cross-system analysis of labile dissolved organic carbon" (PDF). Marine Ecology Progress Series. 118: 283–294. Bibcode:1995MEPS..118..283S. doi:10.3354/meps118283.
- Gruber, David F.; Jean-Paul Simjouw; Sybil P. Seitzinger; Gary L. Taghon (June 2006). "Dynamics and Characterization of Refractory Dissolved Organic Matter Produced by a Pure Bacterial Culture in an Experimental Predator-Prey System". Applied and Environmental Microbiology. 72 (6): 4184–4191. doi:10.1128/AEM.02882-05. PMC 1489638. PMID 16751530.
- Hansell, Dennis A.; Craig A. Carlson; Daniel J. Repeta; Reiner Schlitzer (2009). "Dissolved Organic Matter in the Ocean: A Controversy Stimulates New Insights". Oceanography. 22 (4): 202–211. doi:10.5670/oceanog.2009.109. hdl:1912/3183.
- Kawasaki, N., and Benner, R. (2006). Bacterial release of dissolved organic matter during cell growth and decline: molecular origin and composition. Limnol. Oceanogr. 51, 2170–2180. doi: 10.4319/lo.2006.51.5.2170
- Lønborg, C., Álvarez-Salgado, X. A., Davidson, K., and Miller, A. E. J. (2009). Production of bioavailable and refractory dissolved organic matter by coastal heterotrophic microbial populations. Estuar. Coast. Shelf Sci. 82, 682–688. doi: 10.1016/j.ecss.2009.02.026
- Wada, S., Aoki, M. N., Tsuchiya, Y., Sato, T., Shinagawa, H., and Hama, T. (2007). Quantitative and qualitative analyses of dissolved organic matter released from Ecklonia cava Kjellman, in Oura Bay, Shimoda, Izu Peninsula, Japan. J. Exp. Mar. Biol. Ecol. 349, 344–358. doi: 10.1016/j.jembe.2007.05.024
- Willey, J. D., Kieber, R. J., Eyman, M. S. Jr., and Brooks Avery, G. (2000). Rainwater dissolved organic carbon concentrations and global flux. Glob. Biogeochem. Cycles 14, 139–148. doi: 10.1029/1999GB900036
- Raymond, P. A., and Spencer, R. G. M. (2015). “Riverine DOM,” in Biogeochemistry of Marine Dissolved Organic Matter, eds D. A. Hansell and C. A. Carlson (Amsterdam: Elsevier), 509–533. doi: 10.1016/B978-0-12-405940-5.00011-X
- Dachs, J., and Méjanelle, L. (2010). Organic pollutants in coastal waters, sediments, and biota: a relevant driver for ecosystems during the anthropocene? Estuarines Coasts 33, 1–14. doi: 10.1007/s12237-009-9255-8
- Raymond, P. A., and Spencer, R. G. M. (2015). “Riverine DOM,” in Biogeochemistry of Marine Dissolved Organic Matter, eds D. A. Hansell and C. A. Carlson (Amsterdam: Elsevier), 509–533. doi: 10.1016/B978-0-12-405940-5.00011-X
- Karl, D. M., Hebel, D. V., Bjorkman, K., and Letelier, R. M. (1998). The role of dissolved organic matter release in the productivity of the oligotrophic north Pacific Ocean. Limnol. Oceanogr. 43, 1270–1286. doi: 10.4319/lo.1998.43.6.1270
- Wetz, M. S., and Wheeler, P. A. (2007). Release of dissolved organic matter by coastal diatoms. Limnol. Oceanogr. 52, 798–807. doi: 10.4319/lo.2007.52.2.0798
- Thornton, D. C. O. (2014). Dissolved organic matter (DOM) release by phytoplankton in the contemporary and future ocean. Eur. J. Phycol. 49, 20–46. doi: 10.1080/09670262.2013.875596
- Boekell, W. H. M. V., Hansen, F. C., Riegman, R., and Bak, R. P. M. (1992). Lysis-induced decline of a Phaeocystis spring bloom and coupling with the microbial foodweb. Mar. Ecol. Prog. Ser. 81, 269–276. doi: 10.3354/meps081269
- Hygum, B. H., Petersen, J. W., and Søndergaard, M. (1997). Dissolved organic carbon released by zooplankton grazing activity- a high quality substrate pool for bacteria. J. Plankton Res. 19, 97–111. doi: 10.1093/plankt/19.1.97
- Lampert, W. (1978). Release of dissolved organic carbon by grazing zooplankton. Limnol. Oceanogr. 23, 831–834. doi: 10.4319/lo.1978.23.4.0831
- Jumars, P. A., Penry, D. L., Baross, J. A., and Perry, M. J. (1989). Closing the microbial loop: dissolved carbon pathway to heterotrophic bacteria from incomplete ingestion, digestion and absorption in animals. Deep Sea Res. 36, 483–495. doi: 10.1016/0198-0149(89)90001-0
- Iturriaga, R., and Zsolnay, A. (1981). Transformation of some dissolved organic compounds by a natural heterotrophic population. Mar. Biol. 62, 125–129. doi: 10.1007/BF00388174
- Ogawa, H., Amagai, Y., Koike, I., Kaiser, K., and Benner, R. (2001). Production of refractory dissolved organic matter by bacteria. Science 292, 917–920. doi: 10.1126/science.1057627
- Kawasaki, N., and Benner, R. (2006). Bacterial release of dissolved organic matter during cell growth and decline: molecular origin and composition. Limnol. Oceanogr. 51, 2170–2180. doi: 10.4319/lo.2006.51.5.2170
- McCarthy, M., Pratum, T., Hedges, J., and Benner, R. (1997). Chemical composition of dissolved organic nitrogen in the ocean. Nature 390, 150–154. doi: 10.1038/36535
- Suttle, C. A. (2005). Viruses in the sea. Nature 437, 356–361. doi: 10.1038/nature04160
- Weinbauer, M. A. G. (2004). Ecology of prokaryotic viruses. FEMS Microbiol. Rev. 28, 127–181. doi: 10.1016/j.femsre.2003.08.001
- Lønborg, C., Middelboe, M., and Brussaard, C. P. D. (2013). Viral lysis of Micromonas pusilla: impacts on dissolved organic matter production and composition. Biogeochemistry 116, 231–240. doi: 10.1007/s10533-013-9853-1
- Brilinsky, M. (1977). Release of dissolved organic matter by some marine macrophytes. Mar. Biol. 39, 213–220. doi: 10.1007/BF00390995
- Pregnall, A. M. (1983). Release of dissolved organic carbon from the estuarine intertidal macroalga Enteromorpha prolifera. Mar. Biol. 73, 37–42. doi: 10.1007/BF00396283
- Penhale, P. A., and Smith, W. O. (1977). Excretion of dissolved organic carbon by eelgrass (Zostera marina) and its epiphytes. Limnol. Oceanogr. 22, 400–407. doi: 10.4319/lo.1977.22.3.0400
- Barrón, C., and Duarte, C. M. (2015). Dissolved organic carbon pools and export from the coastal ocean. Glob. Biogeochem. Cycles 29, 1725–1738. doi: 10.1002/2014GB005056
- Barrón, C., and Duarte, C. M. (2015). Dissolved organic carbon pools and export from the coastal ocean. Glob. Biogeochem. Cycles 29, 1725–1738. doi: 10.1002/2014GB005056
- Hewson, I., O’neil, J. M., Fuhrman, J. A., and Dennison, W. C. (2001). Virus-like particle distribution and abundance in sediments and overlying waters along eutrophication gradients in two subtropical estuaries. Limnol. Oceanogr. 46, 1734–1746. doi: 10.4319/lo.2001.46.7.1734
- Burdige, D. J., and Gardner, K. G. (1998). Molecular weight distribution of dissolved organic carbon in marine sediment pore waters. Mar. Chem. 62, 45–64. doi: 10.1016/S0304-4203(98)00035-8
- Burdige, D. J., and Komada, T. (2014). “Sediment pore waters,” in Biogeochemistry of Marine Dissolved Organic Matter, eds D. A. Hansen and C. A. Carlson (Cambridge, MA: Academic Press), 535–577. doi: 10.1016/B978-0-12-405940-5.00012-1
- Komada, T., and Reimers, C. E. (2001). Resuspension-induced partitioning of organic carbon between solid and solution phases from a river–ocean transition. Mar. Chem. 76, 155–174. doi: 10.1016/S0304-4203(01)00055-X
- Dittmar, T., and Koch, B. P. (2006). Thermogenic organic matter dissolved in the abyssal ocean. Mar. Chem. 102, 208–217. doi: 10.1016/j.marchem.2006.04.003
- Dittmar, T., and Paeng, J. (2009). A heat-induced molecular signature in marine dissolved organic matter. Nat. Geosci. 2, 175–179. doi: 10.1038/ngeo440
- Burnett, W. C., Aggarwal, P. K., Aureli, A., Bokuniewicz, H., Cable, J. E., Charette, M. A., et al. (2006). Quantifying submarine groundwater discharge in the coastal zone via multiple methods. Sci. Total Environ. 367, 498–543. doi: 10.1016/j.scitotenv.2006.05.009
- Longnecker, K., and Kujawinski, E. B. (2011). Composition of dissolved organic matter in groundwater. Geochim. Cosmochim. Acta 75, 2752–2761. doi: 10.1016/j.gca.2011.02.020
- Webb, J. R., Santos, I. R., Maher, D. T., Tait, D. R., Cyronak, T., Sadat-Noori, M., et al. (2019). Groundwater as a source of dissolved organic matter to coastal waters: insights from radon and CDOM observations in 12 shallow coastal systems. Limnol. Oceanogr. 64, 182–196. doi: 10.1002/lno.11028
- Lang, S. Q., Butterfield, D. A., Lilley, M. D., Paul Johnson, H., and Hedges, J. I. (2006). Dissolved organic carbon in ridge-axis and ridge-flank hydrothermal systems. Geochim. Cosmochim. Acta 70, 3830–3842. doi: 10.1016/j.gca.2006.04.031
- Kerner, M., Hohenberg, H., Ertl, S., Reckermann, M., and Spitzy, A. (2003). Self-organization of dissolved organic matter tomicelle-like microparticles in river water. Nature 422, 150–154. doi: 10.1038/nature01469
- Chin, W. C., Orellana, M. V., and Verdugo, P. (1998). Spontaneous assembly of marine dissolved organic matter into polymer gels. Nature 391, 568–572. doi: 10.1038/35345
- Moran, M. A., and Zepp, R. G. (1997). Role of photoreactions in the formation of biologically labile compounds from dissolved organic matter. Limnol. Oceanogr. 42, 1307–1316. doi: 10.4319/lo.1997.42.6.1307
- Mopper, K., Kieber, D. J., and Stubbins, A. (2015). “Marine photochemistry of organic matter,” in Biogeochemistry of Marine Dissolved Organic Matter, eds C. A. Carlson and D. A. Hansell (Amsterdam: Elsevier), 389–450. doi: 10.1016/B978-0-12-405940-5.00008-X
- Lønborg, C., and Álvarez-Salgado, X. A. (2012). Recycling versus export of bioavailable dissolved organic matter in the coastal ocean and efficiency of the continental shelf pump. Glob. Biogeochem. Cycles 26:GB3018. doi: 10.1029/2012GB004353
- Carlson, C. A., and Hansell, D. A. (2015). “DOM sources, sinks, reactivity, and budgets,” in Biogeochemistry of Marine Dissolved Organic Matter, eds C. A. Carlson and D. A. Hansell (San Diego, CA: Academic Press), 65–126. doi: 10.1016/B978-0-12-405940-5.00003-0
- Lang, S. Q., Butterfield, D. A., Lilley, M. D., Paul Johnson, H., and Hedges, J. I. (2006). Dissolved organic carbon in ridge-axis and ridge-flank hydrothermal systems. Geochim. Cosmochim. Acta 70, 3830–3842. doi: 10.1016/j.gca.2006.04.031
- Sholkovitz, E. R. (1976). Flocculation of dissolved organic and inorganic matter during the mixing of river water and seawater. Geochim. Cosmochim. Acta 40, 831–845. doi: 10.1016/0016-7037(76)90035-1
- Tranvik, L. J., and Sieburth, J. M. (1989). Effects of flocculated humic matter on free and attached pelagic microorganisms. Limnol. Oceanogr. 34, 688–699. doi: 10.4319/lo.1989.34.4.0688
- Mulholland, P. J. (1981). Formation of Particulate Organic Carbon in Water from a Southeastern Swamp-Stream. Limnol. Oceanogr. 26, 790–795. doi: 10.4319/lo.1981.26.4.0790
- Powell, R. T., Landing, W. M., and Bauer, J. E. (1996). Colloidal trace metals, organic carbon and nitrogen in a southeastern U.S. estuary. Mar. Chem. 55, 165–176. doi: 10.1016/S0304-4203(96)00054-0
- Sholkovitz, E. R., Boyle, E. A., and Price, N. B. (1978). The removal of dissolved humic acids and iron during estuarine mixing. Earth Planet. Sci. Lett. 40, 130–136. doi: 10.1016/0012-821X(78)90082-1
- Volk, C., Bell, K., Ibrahim, E., Verges, D., Amy, G., and Lechevallier, M. (2000). Impact of enhanced and optimized coagulation on removal of organic matter and its biodegradable fraction in drinking water. Water Res. 34, 3247–3257. doi: 10.1016/S0043-1354(00)00033-6
External links
- Stone, Richard (June 18, 2010). "Marine Biogeochemistry: The Invisible Hand Behind A Vast Carbon Reservoir". Science. 328 (5985): 1476–1477. Bibcode:2010Sci...328.1476S. doi:10.1126/science.328.5985.1476. PMID 20558685.