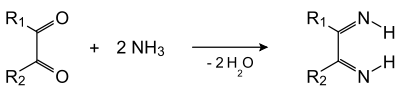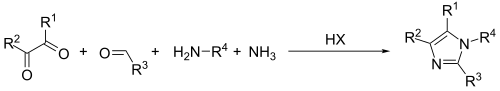Debus-Radziszewski imidazole synthesis
The Debus-Radziszewski imidazole synthesis is an organic reaction used for the synthesis of imidazoles from a dicarbonyl, an aldehyde, and ammonia. The dicarbonyl component is commonly glyoxal, but can also include various 1,2-diketones and ketoaldehydes. The method is used commercially to produce several imidazoles.[1] The process is an example of a multicomponent reaction.
| Debus-Radziszewski imidazole synthesis | |
|---|---|
| Named after | Heinrich Debus Bronisław Leonard Radziszewski |
| Reaction type | Ring forming reaction |
The reaction can be viewed as occurring in two stages. In the first stage, the dicarbonyl and ammonia condense to give a diimine (shown with unusual orientation of N-H groups):
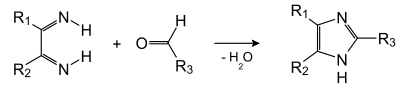
In the second stage, this diimine condenses with the aldehyde:
This reaction is named after Heinrich Debus[2] and Bronisław Leonard Radziszewski.[3][4]
A modification of this general method, where one equivalent of ammonia is replaced by an amine, affords N-substituted imidazoles in good yields.[5]
References
- Ebel, K., Koehler, H., Gamer, A. O., & Jäckh, R. "Imidazole and Derivatives." In Ullmann’s Encyclopedia of Industrial Chemistry; 2002 Wiley-VCH, doi:10.1002/14356007.a13_661
- Debus, Heinrich (1858). "Ueber die Einwirkung des Ammoniaks auf Glyoxal". Justus Liebigs Annalen der Chemie. 107 (2): 199–208. doi:10.1002/jlac.18581070209.
- Radzisewski, Br. (1882). "Ueber Glyoxalin und seine Homologe". Berichte der deutschen chemischen Gesellschaft. 15 (2): 2706–2708. doi:10.1002/cber.188201502245.
- On the development of organic chemistry in Ukraine Dmytro O. Tymoshenko ARKIVOC 2005 (viii) 1-3 Link
- US patent 6,177,575, A. J. Arduengo, "Process for Manufacture of Imidazoles", issued 2001-01-23