Cyclobutanetetrone
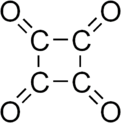
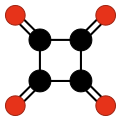
Cyclobutanetetrone, also called tetraoxocyclobutane, is an organic compound[1] with formula C4O4 or (CO)4, the fourfold ketone of cyclobutane. It would be an oxide of carbon, indeed a tetramer of carbon monoxide.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Cyclobutane-1,2,3,4-tetraone | |
| Other names
Tetraoxocyclobutane | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C4O4 | |
| Molar mass | 112.040 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
The compound seems to be thermodynamically unstable.[2] As of 2000, it had yet to be synthesized in significant amounts[3][4] but may have transient existence as detected by mass spectrometry.[5]
Related compounds
Cyclobutanetetrone can be viewed as the neutral counterpart of the squarate anion C
4O2−
4, which is stable and has been known at least since 1959.[6]
The compound octahydroxycyclobutane or cyclobutaneoctaol (C(OH)2)4 may be referred to in the literature as "hydrated tetraoxocyclobutane".[7]
References
- Guo, J.-C.; Hou, G.-L.; Li, S.-D.; Wang, X.-B. (2012). "Probing the Low-Lying Electronic States of Cyclobutanetetraone (C4O4) and Its Radical Anion: A Low-Temperature Anion Photoelectron Spectroscopic Approach". Journal of Physical Chemistry A. 3 (3): 304–308. doi:10.1021/jz201593z.
- Jiao, H.; Frapper, G.; Halet, J.-F.; Saillard, J.-Y. (2001). "Stability of Tetraoxocyclobutane Revised: Perturbation Theory and Density Functional Scheme". Journal of Physical Chemistry Letters. 105 (24): 5945–5947. doi:10.1021/jp010738i.
- Rubin, M. B.; Gleiter, R. (2000). "The Chemistry of Vicinal Polycarbonyl Compounds". Chemical Reviews. 100 (3): 1121–1164. doi:10.1021/cr960079j. PMID 11749259.
- Seitz, G.; Imming, P. (1992). "Oxocarbons and pseudooxocarbons". Chemical Reviews. 92 (6): 1227–1260. doi:10.1021/cr00014a004.
- Schröder, D.; Schwarz, H.; Dua, S.; Blanksby, S. J.; Bowie, J. H. (1999). "Mass spectrometric studies of the oxocarbons CnOn (n = 3–6)". International Journal of Mass Spectrometry. 188 (1–2): 17–25. doi:10.1016/S1387-3806(98)14208-2.
- Cohen, S.; Lacher, J. R.; Park, J. D. (1959). "Diketocyclobutanediol". Journal of the American Chemical Society. 81 (13): 3480. doi:10.1021/ja01522a083.
- Skujins, S.; Delderfield, J.; Webb, G. A. (1967). "A mass spectrometric study of some monocyclic polycarbonyl compounds". Tetrahedron. 24 (13): 4805–4817. doi:10.1016/S0040-4020(01)98676-4.
- Maahs, G.; Hegenberg, P. (2003). "Syntheses and Derivatives of Squaric Acid". Angewandte Chemie International Edition. 5 (10): 888–893. doi:10.1002/anie.196608881.
See also
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.