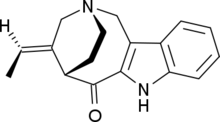
Conolidine
Conolidine is an indole alkaloid. Preliminary reports suggest that it could provide analgesic effects with few of the detrimental side-effects associated with opioids such as morphine, though at present it has only been evaluated in mouse models.
 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C17H18N2O | |
| Molar mass | 266.344 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |

Conolidine was first isolated in 2004 from the bark of the Tabernaemontana divaricata (crepe jasmine) shrub which is used in traditional Chinese medicine.[1]
The first asymmetric total synthesis of conolidine was developed by Micalizio and coworkers in 2011.[2] This synthetic route allows access to either enantiomer (mirror image) of conolidine via an early enzymatic resolution. Notably, evaluation of the synthetic material resulted in the discovery that both enantiomers of the synthetic compound show analgesic effects.[3]
Syntheses
The Micalizio route (2011) achieved the end product in 9 steps from a commercially available acetyl-pyridine. Notable reactions include a [2,3]-Still-Wittig rearrangement and a conformationally-controlled intramolecular Mannich cyclization.
The Weinreb group (2014) used a conjugative addition of an indole precursor to an oxime-substituted nitrosoalkene to generate the tetracyclic skeleton of conolidine in 4 steps.[4]
Takayama and colleagues (2016) synthesized conolidine and apparicine through a gold(I)-catalyzed exo-dig synthesis of a racemic piperidinyl aldehyde.[5]
Ohno and Fujii (2016) accessed the tricyclic pre-Mannich intermediate through a chiral gold(I) catalyzed cascade cyclization.[6]
Pharmacology
In 2011, the Bohn lab noted antinociception against both chemically induced and inflammation-derived pain, and experiments indicated lack of opioid receptor inhibition, but were unable to define a particular target. A 2019 study by a cross-site Australian and U.S. group discovered through cultured neuronal networks that conolidine may inhibit the Ca v2.2 channel, a mechanism seen in molecules like conotoxin. The group was unable to rule out partial polypharmacology against other targets.[7]
See also
References
- Kam, T.-S.; Pang, H. S.; Choo, Y. M.; Komiyama, K. (Apr 2004). "Biologically Active Ibogan and Vallesamine Derivatives from Tabernaemontana divaricata". Chemistry & Biodiversity. 1 (4): 646–656. doi:10.1002/cbdv.200490056. PMID 17191876.
- Tarselli, M. A.; Raehal, K. M.; Brasher, A. K.; Groer, C.; Cameron, M. D.; Bohn, L. M.; Micalizio, G. C. (2011). "Synthesis of Conolidine, a Potent Non-Opioid Analgesic for Tonic and Persistent Pain". Nature Chemistry. 3 (6): 449–453. doi:10.1038/nchem.1050. PMID 21602859.
- Ball, P. (May 2011). "Compound Offers Pain Relief Without the Complications". Nature. doi:10.1038/news.2011.313.
- Chauhan, P.S.; Weinreb, S.M. (2014). "Convergent Approach to the Tetracyclic Core of the Apparicine Class of Indole Alkaloids via a Key Intermolecular Nitrosoalkene Conjugate Addition". Journal of Organic Chemistry. 79 (13): 6389–6393. doi:10.1021/jo501067u.
- Takanashi, N.; Suzuki, K.; Kitajima, M.; Takayama, H. (2016). "Total Synthesis of Conolidine and Apparicine". Tetrahedron Letters. 57 (3): 375–378. doi:10.1016/j.tetlet.2015.12.029.
- Noae, S.; Yoshida, Y.; Oishi, S.; Fujii, N.; Ohno, H. (2016). "Total Synthesis of (+)-Conolidine by the Gold(I)-Catalyzed Cascade Cyclization of a Conjugated Enyne" (PDF). Journal of Organic Chemistry. 81 (13): 5690–5698. doi:10.1021/acs.joc.6b00720. hdl:2433/241631.
- Petrou, S.; Halgamuge, S.; Reid, C. A.; Osborne, P. B.; M. Varney; Li, M.; Pachernegg, S.; Morrisroe, E.; Berecki, G. (2019-01-15). "Discovering the pharmacodynamics of conolidine and cannabidiol using a cultured neuronal network based workflow". Scientific Reports. 9 (1): 121. doi:10.1038/s41598-018-37138-w. ISSN 2045-2322. PMC 6333801. PMID 30644434.