Chromyl chloride
Chromyl chloride is a chemical compound with the formula CrO2Cl2. The molecule is tetrahedral, like most of the commonly encountered chromium(VI) derivatives such as chromate, [CrO4]2−. In terms of physical properties and structure, it resembles sulfuryl chloride. It is a hygroscopic dark red liquid, that in terms of color and volatility has also been described as resembling bromine.[3]
| |||
 | |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Chromium(VI) dichloride dioxide | |||
| Systematic IUPAC name
Dichlorodioxochromium | |||
| Other names
Chromic acid chloride Chromium oxychloride Chlorochromic anhydride Chromic oxychloride Chromium chloride oxide Chromium dioxide dichloride Chromium dioxychloride Chromium oxychloride | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.035.491 | ||
| EC Number |
| ||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| CrO2Cl2 | |||
| Molar mass | 154.9008 g/mol | ||
| Appearance | blood red fuming liquid, similar to bromine | ||
| Odor | musty, burning, acrid[1] | ||
| Density | 1.911 g/mL, liquid | ||
| Melting point | -96.5 °C | ||
| Boiling point | 117 °C | ||
| Very soluble,hydrolysis | |||
| Vapor pressure | 20 mmHg (20 °C)[1] | ||
| Hazards | |||
| Main hazards | carcinogen, reacts violently with water[1] | ||
| GHS pictograms | 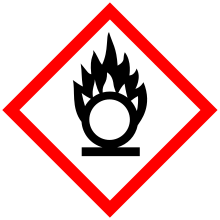 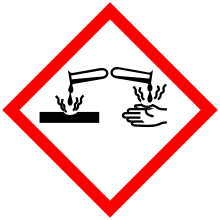     | ||
| NFPA 704 (fire diamond) | |||
| Flash point | noncombustible [1] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible) |
none[1] | ||
REL (Recommended) |
Ca TWA 0.001 mg Cr(VI)/m3[1] | ||
IDLH (Immediate danger) |
N.D.[1] | ||
| Related compounds | |||
Related compounds |
SO2Cl2; VOCl3; MoO2Cl2; WO2Cl2; CrO2F2 | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Preparation
Chromyl chloride can be prepared by the reaction of potassium chromate or potassium dichromate with hydrogen chloride in the presence of sulfuric acid, followed by gentle distillation.[4][3]
- K2Cr2O7 + 6 HCl → 2 CrO2Cl2 + 2 KCl + +3 H2O
The sulfuric acid serves as the dehydration agent. It can also be prepared directly by exposing chromium trioxide to anhydrous hydrogen chloride gas.
- CrO3 + 2 HCl ⇌ CrO2Cl2 + H2O
The method used to prepare chromyl chloride is the basis for a qualitative test for chloride: a sample suspected of containing chloride is heated with a mixture of potassium dichromate and concentrated sulfuric acid. If chloride is present, chromyl chloride forms as evidenced by red fumes of CrO2Cl2. Analogous compounds are not formed with fluorides, bromides, iodides and cyanides.
Reagent for oxidation of alkenes
Chromyl chloride oxidizes internal alkenes to alpha-chloroketones or related derivatives.[5] It will also attack benzylic methyl groups to give aldehydes via the Étard reaction. Dichloromethane is a suitable solvent for these reactions.[6]
Safety considerations
CrO2Cl2 hydrolyzes to release hydrochloric acid (HCl) and hexavalent chromium (CrVI)
Acute: Exposure to chromyl chloride vapour irritates the respiratory system and severely irritates the eyes, and the liquid burns the skin and eyes. Ingestion would cause severe internal damage.[7]
Chronic: CrVI can produce chromosomal aberrations and is a human carcinogen via inhalation.[8] Frequent exposure of the skin to chromyl chloride may result in ulceration.[7]
References
| Wikimedia Commons has media related to Chromyl chloride. |
- NIOSH Pocket Guide to Chemical Hazards. "#0142". National Institute for Occupational Safety and Health (NIOSH).
- Sisler, Harry H. (1946). "Chromyl Chloride [Chromium(VI) Dioxychloride]". Inorganic Syntheses. 2: 205–207. doi:10.1002/9780470132333.ch63.
- Moody, B.J. (1965). "22". Comparative Inorganic Chemistry (1 ed.). London: Edward Arnold. p. 381. ISBN 0-7131-3679-0.
- Freeman, Fillmore; DuBois, Richard H.; McLaughlin, Thomas G. (1971). "Aldehydes by Oxidation of Terminal Olefins with Chromyl Chloride: 2,4,4-Trimethylpentanal". Org. Synth. 51: 4. doi:10.15227/orgsyn.051.0004.
- F. Freeman (2004). "Chromyl Chloride". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rc177..}}
- Prof CH Gray, ed. (1966). "IV". Laboratory Handbook of Toxic Agents (2 ed.). London: Royal Institute of Chemistry. p. 79.
- IARC (1999-11-05) [1990]. Volume 49: Chromium, Nickel, and Welding (PDF). pp. 21–23. ISBN 92-832-1249-5. Archived from the original (PDF) on 2008-12-24. Retrieved 2008-03-26.


