Channelrhodopsin
Channelrhodopsins are a subfamily of retinylidene proteins (rhodopsins) that function as light-gated ion channels.[1] They serve as sensory photoreceptors in unicellular green algae, controlling phototaxis: movement in response to light.[2] Expressed in cells of other organisms, they enable light to control electrical excitability, intracellular acidity, calcium influx, and other cellular processes (see optogenetics). Channelrhodopsin-1 (ChR1) and Channelrhodopsin-2 (ChR2) from the model organism Chlamydomonas reinhardtii are the first discovered channelrhodopsins. Variants have been cloned from other algal species, and more are expected.
Structure
In terms of structure, channelrhodopsins are retinylidene proteins. They are seven-transmembrane proteins like rhodopsin, and contain the light-isomerizable chromophore all-trans-retinal (an aldehyde derivative of vitamin A). The retinal chromophore is covalently linked to the rest of the protein through a protonated Schiff base. Whereas most 7-transmembrane proteins are G protein-coupled receptors that open other ion channels indirectly via second messengers (i.e., they are metabotropic), channelrhodopsins directly form ion channels (i.e., they are ionotropic).[4] This makes cellular depolarization extremely fast, robust, and useful for bioengineering and neuroscience applications, including photostimulation.
Function
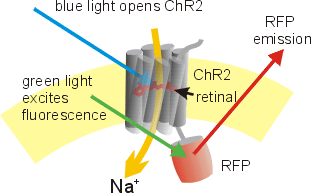
The natural ("wild-type") ChR2 absorbs blue light with an absorption and action spectrum maximum at 480 nm.[5] When the all-trans-retinal complex absorbs a photon, it induces a conformational change from all-trans to 13-cis-retinal. This change introduces a further one in the transmembrane protein, opening the pore to at least 6 Å. Within milliseconds, the retinal relaxes back to the all-trans form, closing the pore and stopping the flow of ions.[4] Most natural channelrhodopsins are nonspecific cation channels, conducting H+, Na+, K+, and Ca2+ ions. Recently, anion-conducting channelrhodopsins have been discovered.[6]
Designer-channelrhodopsins
Channelrhodopsins are key tools in optogenetics. The C-terminal end of Channelrhodopsin-2 extends into the intracellular space and can be replaced by fluorescent proteins without affecting channel function. This kind of fusion construct can be useful to visualize the morphology of ChR2 expressing cells.[7][8] Point mutations close to the retinal binding pocket have been shown to affect the biophysical properties of the channelrhodopsin, resulting in a variety of different tools.
Kinetics
Closing of the channel after optical activation can be substantially delayed by mutating the protein residues C128 or D156. This modification results in super-sensitive channelrhodopsins that can be opened by a blue light pulse and closed by a green or yellow light pulse (Step-function opsins).[9][10][11] Mutating the E123 residue accelerates channel kinetics (ChETA), and the resulting ChR2 mutants have been used to spike neurons at up to 200 Hz.[12] In general, channelrhodopsins with slow kinetics are more light-sensitive on the population level, as open channels accumulate over time even at low light levels.
Photocurrent amplitude
H134R and T159C mutants display increased photocurrents, and a combination of T159 and E123 (ET/TC) has slightly larger photocurrents and slightly faster kinetics than wild-type ChR2.[13] Among ChR variants, ChIEF, a chimera and point mutant of ChR1 and ChR2, demonstrates the largest photocurrents and the least desensitization and has kinetics similar to wild-type ChR2.[14]
Wavelength
Chimeric channelrhodopsins have been developed by combining transmembrane helices from ChR1 and VChR1, leading to the development of ChRs with red spectral shifts (such as C1V1 and ReaChR).[11][15] ReaChR has improved membrane trafficking and strong expression in mammalian cells, and has been used for minimally invasive, transcranial activation of brainstem motoneurons. Searches for homologous sequences in other organisms has yielded spectrally improved and stronger red-shifted channelrhodpsins (Chrimson).[16] In combination with ChR2, these yellow/red light-sensitive channelrhodopsins allow controlling two populations of neurons independently with light pulses of different colors.[17]
A blue-shifted channelrhodopsin has been discovered in the alga Scherffelia dubia. After some engineering to improve membrane trafficking and speed, the resulting tool (CheRiff) produced large photocurrents at 460 nm excitation.[18] It has been combined with the Genetically Encoded Calcium Indicator jRCaMP1b [19] in an all-optical system called the OptoCaMP.[20]
Ion selectivity
The L132C mutation (CatCh) increases the permeability for calcium and generates very large currents.[21] Mutating E90 to the positively charged amino acid arginine turns channelrhodopsin from an unspecific cation channel into a chloride-conducting channel (ChloC).[22] The selectivity for Cl- was further improved by replacing negatively charged residues in the channel pore, making the reversal potential more negative.[23][24] Selective chloride-conducting channelrhodopsins (iChloC, iC++, GtACR) inhibit neuronal spiking in cell culture and in intact animals when illuminated with blue light.
Applications
Channelrhodopsins can be readily expressed in excitable cells such as neurons using a variety of transfection techniques (viral transfection, electroporation, gene gun) or transgenic animals. The light-absorbing pigment retinal is present in most cells (of vertebrates) as Vitamin A, making it possible to photostimulate neurons without adding any chemical compounds. Before the discovery of channelrhodopsins, neuroscientists were limited to recording the activity of neurons in the brain and correlate this activity with behavior. This is not sufficient to prove that the recorded neural activity actually caused that behavior. Controlling networks of genetically modified cells with light, an emerging field known as Optogenetics., allows researchers now to explore the causal link between activity in a specific group of neurons and mental events, e.g. decision making. Optical control of behavior has been demonstrated in nematodes, fruit flies, zebrafish, and mice.[25][26] Recently, chloride-conducting channelrhodopsins have been engineered and were also found in nature.[6][22] These tools can be used to silence neurons in cell culture and in live animals by shunting inhibition.[23][24]
Using multiple colors of light expands the possibilities of optogenetic experiments. The blue-light sensitive ChR2 and the yellow light-activated chloride pump halorhodopsin together enable multiple-color optical activation and silencing of neural activity.[27][28] VChR1 from the colonial alga Volvox carteri absorbs maximally at 535 nm and had been used to stimulate cells with yellow light (580 nm), although photocurrents generated by VChR1 are typically very small.[29] However, VChR1-ChR2 hybrids have been developed using directed evolution that display maximal excitation at 560 nm, and 50% of peak absorbance at wavelengths over 600 nm.[15][30]
Using fluorescently labeled ChR2, light-stimulated axons and synapses can be identified.[8] This is useful to study the molecular events during the induction of synaptic plasticity.[31] Transfected cultured neuronal networks can be stimulated to perform some desired behaviors for applications in robotics and control.[32] ChR2 has also been used to map long-range connections from one side of the brain to the other, and to map the spatial location of inputs on the dendritic tree of individual neurons.[33][34]
Visual function in blind mice can be partially restored by expressing ChR2 in inner retinal cells.[35][36] In the future, ChR2 might find medical applications, e.g. in forms of retinal degeneration or for deep-brain stimulation. Optical cochlear implants have been shown to work well in animal experiments and might lead to the first application of optogenetics in human patients.[37][38][39]
History
Motility and photoorientation of microalgae (phototaxis) have been studied over more than hundred years in many laboratories worldwide.
In 1980, Ken Foster developed the first consistent theory about the functionality of algal eyes.[40] He also analyzed published action spectra and complemented blind cells with retinal and retinal analogues, which led to the conclusion that the photoreceptor for motility responses in Chlorophyceae is rhodopsin.[41]
Photocurrents of the Chlorophyceae Heamatococcus pluvialis and Chlamydomonas reinhardtii were studied over many years in the groups of Oleg Sineshchekov and Peter Hegemann, resulting in two seminal publications in the years 1978 and 1991.[42][43] Based on action spectroscopy and simultaneous recordings of photocurrents and flagellar beating, it was determined that the photoreceptor currents and subsequent flagellar movements are mediated by rhodopsin and control phototaxis and photophobic responses. The extremely fast rise of the photoreceptor current after a brief light flash led to the conclusion that the rhodopsin and the channel are intimately linked in a protein complex, or even within one single protein.[44][45]
However, biochemical purification of the rhodopsin-photoreceptor(s) was unsuccessful for many years.
The nucleotide sequences of the rhodopsins now called channelrhodopsins ChR1 and ChR2 were finally uncovered in a large-scale EST sequencing project in C. reinhardtii. Independent submission of the same sequences to GenBank by three research groups generated confusion about their naming: The names cop-3 and cop-4 were used for initial submission by Hegemann's group;[46] csoA and csoB by Spudich's group;[2] and acop-1 and acop-2 by Takahashi's group.[47] Both sequences were found to function as single-component light-activated cation channels in a Xenopus oocytes and human kidney cells (HEK) by Georg Nagel, Ernst Bamberg, Peter Hegemann and others.[1][4]
The name "channelrhodopsin" was coined to highlight this unusual property, and the sequences were renamed accordingly. Meanwhile, their roles in generation of photoreceptor currents in algal cells were characterized by Oleg Sineshchekov, Kwang-Hwan Jung and John Spudich,[2] and Peter Berthold and Peter Hegemann.[48]
In November 2004, Zhuo-Hua Pan submitted a paper to Nature reporting restoration of eyesight in blind mice transfected with Channelrhodopsin, but the paper was rejected and ultimately published in Neuron in 2006.
Meanwhile, in 2005, three groups sequentially established ChR2 as a tool for genetically targeted optical remote control (optogenetics) of neurons, neural circuits and behavior.
At first, Karl Deisseroth's lab (in a paper published in August 2005) demonstrated that ChR2 could be deployed to control mammalian neurons in vitro, achieving temporal precision on the order of milliseconds (both in terms of delay to spiking and in terms of temporal jitter).[7] This was a significant finding, since, first, all opsins (microbial as well as vertebrate) require retinal as the light-sensing co-factor and it was unclear whether central mammalian nerve cells would contain sufficient retinal levels, but they do; second, it showed, despite the small single-channel conductance, sufficient potency to drive mammalian neurons above action potential threshold; and, third, it demonstrated channelrhodopsin to be the first optogenetic tool, with which neural activity could be controlled with the temporal precision at which neurons operate (milliseconds). An earlier tool for photostimulation, cHARGe, demonstrated proof of principle in cultured neurons[49] but was never used by other groups since it operated with a precision on the order of seconds, was highly variable, and did not allow control of individual action potentials.
A second study was published later by Peter Hegemann's and Stefan Herlitze's groups confirming the ability of ChR2 to control the activity of vertebrate neurons, at this time in the chick spinal cord.[50] This study was the first wherein ChR2 was expressed alongside an optical silencer, vertebrate rhodopsin-4 in this case, demonstrating for the first time that excitable cells could be activated and silenced using these two tools simultaneously, illuminating the tissue at different wavelengths.
The groups of Alexander Gottschalk and Ernst Bamberg (with Georg Nagel taking the experimental lead) demonstrated that ChR2, if expressed in specific neurons or muscle cells, can evoke predictable behaviors, i.e. can control the nervous system of an intact animal, in this case the invertebrate C. elegans.[51] This was the first using ChR2 to steer the behavior of an animal in an optogenetic experiment, rendering a genetically specified cell type subject to optical remote control. Although both aspects had been illustrated earlier that year by another group, the Miesenböck lab, deploying the indirectly light-gated ion channel P2X2,[52] it was henceforth microbial opsins like channelrhodopsin that dominated the field of genetically targeted remote control of excitable cells, due to the power, speed, targetability, ease of use, and temporal precision of direct optical activation, not requiring any external chemical compound such as caged ligands.[53]
To overcome its principal downsides — the small single-channel conductance (especially in steady-state), the limitation to one optimal excitation wavelength (~470 nm, blue) as well as the relatively long recovery time, not permitting controlled firing of neurons above 20–40 Hz — ChR2 has been optimized using genetic engineering. A point mutation H134R (exchanging the amino acid Histidine in position 134 of the native protein for an Arginine) resulted in increased steady-state conductance, as described in a 2005 paper that also established ChR2 as an optogenetic tool in C. elegans.[51] In 2009, Roger Tsien's lab optimized ChR2 for further increases in steady-state conductance and dramatically reduced desensitization by creating chimeras of ChR1 and ChR2 and mutating specific amino acids, yielding ChEF and ChIEF, which allowed the driving of trains of action potentials up to 100 Hz.[14][54] In 2010, the groups of Hegemann and Deisseroth introduced an E123T mutation into native ChR2, yielding ChETA, which has faster on- and off-kinetics, permitting the control of individual action potentials at frequencies up to 200 Hz (in appropriate cell types).[12][14]
The groups of Hegemann and Deisseroth also discovered that the introduction of the point mutation C128S makes the resulting ChR2-derivative a step-function tool: Once "switched on" by blue light, ChR2(C128S) stays in the open state until it is switched off by yellow light – a modification that deteriorates temporal precision, but increases light sensitivity by two orders of magnitude.[9] They also discovered and characterized VChR1 in the multicellular algae Volvox carteri. VChR1 produces only tiny photocurrents, but with an absorption spectrum that is red-shifted relative to ChR2.[29] Using parts of the ChR1 sequence, photocurrent amplitude was later improved to allow excitation of two neuronal populations at two distinct wavelengths.[11]
Deisseroth's group has pioneered many applications in live animals such as genetically targeted remote control in rodents in vivo,[55] the optogenetic induction of learning in rodents,[56] the experimental treatment of Parkinson's disease in rats,[57][58] and the combination with fMRI (opto-fMRI).[59] Other labs have pioneered the combination of ChR2 stimulation with calcium imaging for all-optical experiments,[8] mapping of long-range[33] and local[60] neural circuits, ChR2 expression from a transgenic locus – directly[61] or in the Cre-lox conditional paradigm[60] – as well as the two-photon excitation of ChR2, permitting the activation of individual cells.[62][63][64]
In March 2013, the Brain Prize (Grete Lundbeck European Brain Research Prize) was jointly awarded to Bamberg, Boyden, Deisseroth, Hegemann, Miesenböck, and Nagel for "their invention and refinement of optogenetics".[65] The same year, Hegemann and Nagel received the Louis-Jeantet Prize for Medicine for "the discovery of channelrhodopsin". In 2015, Boyden and Deisseroth received the Breakthrough Prize in Life Sciences and in 2020, Miesenböck, Hegemann and Nagel received the Shaw prize in Life Science and Medicine for the development of optogenetics.
References
- Nagel G, Ollig D, Fuhrmann M, Kateriya S, Musti AM, Bamberg E, Hegemann P (June 2002). "Channelrhodopsin-1: a light-gated proton channel in green algae". Science. 296 (5577): 2395–8. doi:10.1126/science.1072068. PMID 12089443.
- Sineshchekov OA, Jung KH, Spudich JL (June 2002). "Two rhodopsins mediate phototaxis to low- and high-intensity light in Chlamydomonas reinhardtii". Proc. Natl. Acad. Sci. U.S.A. 99 (13): 8689–94. doi:10.1073/pnas.122243399. PMC 124360. PMID 12060707.
- Kato HE, Zhang F, Yizhar O, Ramakrishnan C, Nishizawa T, Hirata K, Ito J, Aita Y, Tsukazaki T, Hayashi S, Hegemann P, Maturana AD, Ishitani R, Deisseroth K, Nureki O (February 2012). "Crystal structure of the channelrhodopsin light-gated cation channel". Nature. 482 (7385): 369–74. doi:10.1038/nature10870. PMC 4160518. PMID 22266941.
- Nagel G, Szellas T, Huhn W, Kateriya S, Adeishvili N, Berthold P, Ollig D, Hegemann P, Bamberg E (November 2003). "Channelrhodopsin-2, a directly light-gated cation-selective membrane channel". Proc. Natl. Acad. Sci. U.S.A. 100 (24): 13940–5. doi:10.1073/pnas.1936192100. PMC 283525. PMID 14615590.
- Bamann C, Kirsch T, Nagel G, Bamberg E (January 2008). "Spectral characteristics of the photocycle of channelrhodopsin-2 and its implication for channel function". J. Mol. Biol. 375 (3): 686–94. doi:10.1016/j.jmb.2007.10.072. PMID 18037436.
- Govorunova EG, Sineshchekov OA, Janz R, Liu X, Spudich JL (2015). "Natural light-gated anion channels: A family of microbial rhodopsins for advanced optogenetics". Science. 349 (6248): 647–650. doi:10.1126/science.aaa7484. PMC 4764398. PMID 26113638.
- Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K (September 2005). "Millisecond-timescale, genetically targeted optical control of neural activity". Nat. Neurosci. 8 (9): 1263–8. doi:10.1038/nn1525. PMID 16116447.
- Zhang YP, Oertner TG (February 2007). "Optical induction of synaptic plasticity using a light-sensitive channel". Nat. Methods. 4 (2): 139–41. doi:10.1038/nmeth988. PMID 17195846.
- Berndt A, Yizhar O, Gunaydin LA, Hegemann P, Deisseroth K (February 2009). "Bi-stable neural state switches". Nat. Neurosci. 12 (2): 229–34. doi:10.1038/nn.2247. PMID 19079251.
- Schoenenberger P, Gerosa D, Oertner TG (2009). "Temporal control of immediate early gene induction by light". PLoS ONE. 4 (12): e8185. doi:10.1371/journal.pone.0008185. PMC 2780714. PMID 19997631.
- Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O'Shea DJ, Sohal VS, Goshen I, Finkelstein J, Paz JT, Stehfest K, Fudim R, Ramakrishnan C, Huguenard JR, Hegemann P, Deisseroth K (September 2011). "Neocortical excitation/inhibition balance in information processing and social dysfunction". Nature. 477 (7363): 171–8. doi:10.1038/nature10360. PMC 4155501. PMID 21796121.
- Gunaydin LA, Yizhar O, Berndt A, Sohal VS, Deisseroth K, Hegemann P (March 2010). "Ultrafast optogenetic control". Nat. Neurosci. 13 (3): 387–92. doi:10.1038/nn.2495. PMID 20081849.
- Berndt A, Schoenenberger P, Mattis J, Tye KM, Deisseroth K, Hegemann P, Oertner TG (May 2011). "High-efficiency channelrhodopsins for fast neuronal stimulation at low light levels". Proceedings of the National Academy of Sciences of the United States of America. 108 (18): 7595–600. doi:10.1073/pnas.1017210108. PMC 3088623. PMID 21504945.
- Lin JY (January 2011). "A user's guide to channelrhodopsin variants: features, limitations and future developments". Experimental Physiology. 96 (1): 19–25. doi:10.1113/expphysiol.2009.051961. PMC 2995811. PMID 20621963.
- Lin JY, Knutsen PM, Muller A, Kleinfeld D, Tsien RY (October 2013). "ReaChR: a red-shifted variant of channelrhodopsin enables deep transcranial optogenetic excitation". Nature Neuroscience. 16 (10): 1499–508. doi:10.1038/nn.3502. PMC 3793847. PMID 23995068.
- Klapoetke NC, Murata Y, Kim SS, Pulver SR, Birdsey-Benson A, Cho YK, Morimoto TK, Chuong AS, Carpenter EJ, Tian Z, Wang J, Xie Y, Yan Z, Zhang Y, Chow BY, Surek B, Melkonian M, Jayaraman V, Constantine-Paton M, Wong GK, Boyden ES (March 2014). "Independent optical excitation of distinct neural populations". Nature Methods. 11 (3): 338–46. doi:10.1038/nmeth.2836. PMC 3943671. PMID 24509633.
- Hooks BM, Lin JY, Guo C, Svoboda K (March 2015). "Dual-channel circuit mapping reveals sensorimotor convergence in the primary motor cortex". The Journal of Neuroscience. 35 (10): 4418–26. doi:10.1523/JNEUROSCI.3741-14.2015. PMC 4355205. PMID 25762684.
- Hochbaum DR, Zhao Y, Farhi SL, Klapoetke N, Werley CA, Kapoor V, Zou P, Kralj JM, Maclaurin D, Smedemark-Margulies N, Saulnier JL, Boulting GL, Straub C, Cho YK, Melkonian M, Wong GK, Harrison DJ, Murthy VN, Sabatini BL, Boyden ES, Campbell RE, Cohen AE (August 2014). "All-optical electrophysiology in mammalian neurons using engineered microbial rhodopsins". Nature Methods. 11 (8): 825–33. doi:10.1038/nmeth.3000. PMC 4117813. PMID 24952910.
- Dana H, Mohar B, Sun Y, Narayan S, Gordus A, Hasseman JP, Tsegaye G, Holt GT, Hu A, Walpita D, Patel R, Macklin JJ, Bargmann CI, Ahrens MB, Schreiter ER, Jayaraman V, Looger LL, Svoboda K, Kim DS (March 2016). "Sensitive red protein calcium indicators for imaging neural activity". eLife. 5. doi:10.7554/eLife.12727. PMC 4846379. PMID 27011354.
- Afshar Saber W, Gasparoli FM, Dirks MG, Gunn-Moore FJ, Antkowiak M (2018). "All-Optical Assay to Study Biological Neural Networks". Frontiers in Neuroscience. 12: 451. doi:10.3389/fnins.2018.00451. PMC 6041400. PMID 30026684.
- Kleinlogel S, Feldbauer K, Dempski RE, Fotis H, Wood PG, Bamann C, Bamberg E (April 2011). "Ultra light-sensitive and fast neuronal activation with the Ca²+-permeable channelrhodopsin CatCh" (PDF). Nature Neuroscience. 14 (4): 513–8. doi:10.1038/nn.2776. PMID 21399632.
- Wietek J, Wiegert JS, Adeishvili N, Schneider F, Watanabe H, Tsunoda SP, Vogt A, Elstner M, Oertner TG, Hegemann P (April 2014). "Conversion of channelrhodopsin into a light-gated chloride channel". Science. 344 (6182): 409–12. doi:10.1126/science.1249375. PMID 24674867.
- Wietek J, Beltramo R, Scanziani M, Hegemann P, Oertner TG, Wiegert JS (October 2015). "An improved chloride-conducting channelrhodopsin for light-induced inhibition of neuronal activity in vivo". Scientific Reports. 5: 14807. doi:10.1038/srep14807. PMC 4595828. PMID 26443033.
- Berndt A, Lee SY, Wietek J, Ramakrishnan C, Steinberg EE, Rashid AJ, Kim H, Park S, Santoro A, Frankland PW, Iyer SM, Pak S, Ährlund-Richter S, Delp SL, Malenka RC, Josselyn SA, Carlén M, Hegemann P, Deisseroth K (January 2016). "Structural foundations of optogenetics: Determinants of channelrhodopsin ion selectivity". Proceedings of the National Academy of Sciences of the United States of America. 113 (4): 822–9. doi:10.1073/pnas.1523341113. PMC 4743797. PMID 26699459.
- Douglass AD, Kraves S, Deisseroth K, Schier AF, Engert F (August 2008). "Escape behavior elicited by single, channelrhodopsin-2-evoked spikes in zebrafish somatosensory neurons". Curr. Biol. 18 (15): 1133–7. doi:10.1016/j.cub.2008.06.077. PMC 2891506. PMID 18682213.
- Huber D, Petreanu L, Ghitani N, Ranade S, Hromádka T, Mainen Z, Svoboda K (January 2008). "Sparse optical microstimulation in barrel cortex drives learned behaviour in freely moving mice". Nature. 451 (7174): 61–4. doi:10.1038/nature06445. PMC 3425380. PMID 18094685.
- Han X, Boyden ES (2007). "Multiple-color optical activation, silencing, and desynchronization of neural activity, with single-spike temporal resolution". PLoS ONE. 2 (3): e299. doi:10.1371/journal.pone.0000299. PMC 1808431. PMID 17375185.
- Zhang F, Wang LP, Brauner M, Liewald JF, Kay K, Watzke N, Wood PG, Bamberg E, Nagel G, Gottschalk A, Deisseroth K (April 2007). "Multimodal fast optical interrogation of neural circuitry". Nature. 446 (7136): 633–9. doi:10.1038/nature05744. PMID 17410168.
- Zhang F, Prigge M, Beyrière F, Tsunoda SP, Mattis J, Yizhar O, Hegemann P, Deisseroth K (June 2008). "Red-shifted optogenetic excitation: a tool for fast neural control derived from Volvox carteri". Nat. Neurosci. 11 (6): 631–3. doi:10.1038/nn.2120. PMC 2692303. PMID 18432196.
- Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O'Shea DJ, Sohal VS, Goshen I, Finkelstein J, Paz JT, Stehfest K, Fudim R, Ramakrishnan C, Huguenard JR, Hegemann P, Deisseroth K (July 2011). "Neocortical excitation/inhibition balance in information processing and social dysfunction". Nature. 477 (7363): 171–8. doi:10.1038/nature10360. PMC 4155501. PMID 21796121.
- Zhang YP, Holbro N, Oertner TG (August 2008). "Optical induction of plasticity at single synapses reveals input-specific accumulation of alphaCaMKII". Proc. Natl. Acad. Sci. U.S.A. 105 (33): 12039–44. doi:10.1073/pnas.0802940105. PMC 2575337. PMID 18697934.
- Xu Z, Ziye X, Craig H, Silvia F (Dec 2013). Spike-based indirect training of a spiking neural network-controlled virtual insect. IEEE Decision and Control. pp. 6798–6805. CiteSeerX 10.1.1.671.6351. doi:10.1109/CDC.2013.6760966. ISBN 978-1-4673-5717-3.
- Petreanu L, Huber D, Sobczyk A, Svoboda K (May 2007). "Channelrhodopsin-2-assisted circuit mapping of long-range callosal projections". Nat. Neurosci. 10 (5): 663–8. doi:10.1038/nn1891. PMID 17435752.
- Petreanu L, Mao T, Sternson SM, Svoboda K (February 2009). "The subcellular organization of neocortical excitatory connections". Nature. 457 (7233): 1142–5. doi:10.1038/nature07709. PMC 2745650. PMID 19151697.
- Bi A, Cui J, Ma YP, Olshevskaya E, Pu M, Dizhoor AM, Pan ZH (April 2006). "Ectopic expression of a microbial-type rhodopsin restores visual responses in mice with photoreceptor degeneration". Neuron. 50 (1): 23–33. doi:10.1016/j.neuron.2006.02.026. PMC 1459045. PMID 16600853.
- Lagali PS, Balya D, Awatramani GB, Münch TA, Kim DS, Busskamp V, Cepko CL, Roska B (June 2008). "Light-activated channels targeted to ON bipolar cells restore visual function in retinal degeneration". Nat. Neurosci. 11 (6): 667–75. doi:10.1038/nn.2117. PMID 18432197.
- Hernandez VH, Gehrt A, Reuter K, Jing Z, Jeschke M, Mendoza Schulz A, Hoch G, Bartels M, Vogt G, Garnham CW, Yawo H, Fukazawa Y, Augustine GJ, Bamberg E, Kügler S, Salditt T, de Hoz L, Strenzke N, Moser T (Feb 2014). "Optogenetic stimulation of the auditory pathway". J Clin Invest. 124 (3): 1114–29. doi:10.1172/JCI69050. PMC 3934189. PMID 24509078.
- Mager T, Lopez de la Morena D, Senn V, Schlotte J, D'Errico A, Feldbauer K, Wrobel C, Jung S, Bodensiek K, Rankovic V, Browne L, Huet A, Jüttner J, Wood PG, Letzkus JJ, Moser T, Bamberg E (May 2018). "High frequency neural spiking and auditory signaling by ultrafast red-shifted optogenetics". Nat Commun. 9 (1): 1750. doi:10.1038/s41467-018-04146-3. PMC 5931537. PMID 29717130.
- Keppeler D, Martins Merino R, Lopez de la Morena D, Bali B, Huet AT, Gehrt A, Wrobel C, Subramanian S, Dombrowski T, Wolf F, Rankovic V, Neef A, Moser T (2018). "Ultrafast optogenetic stimulation of the auditory pathway by targeting‐optimized Chronos". EMBO J. 37 (24): e99649. doi:10.15252/embj.201899649. PMC 6293277. PMID 30396994.
- Foster KW, Smyth R (1980). "Light Antennas in Phototactic Algae". Microbiological Reviews. 44 (4): 572–630. PMC 373196. PMID 7010112.
- Foster KW, Saranak J, Patel N, Zarilli G, Okabe M, Kline T, Nakanishi K (October 1984). "A rhodopsin is the functional photoreceptor for phototaxis in the unicellular eukaryote Chlamydomonas". Nature. 311 (5988): 489–491. doi:10.1038/311756a0. PMID 6493336.
- Litvin FF, Sineshchekov OA, Sineshchekov VA (1978). "Photoreceptor electric potential in the phototaxis of the alga Haematococcus pluvialis". Nature. 271 (5644): 476–478. doi:10.1038/271476a0. PMID 628427.
- Harz H, Hegemann P (June 1991). "Rhodopsin-regulated calcium currents in Chlamydomonas". Nature. 351 (6326): 489–491. doi:10.1038/351489a0.
- Holland EM, Braun FJ, Nonnengässer C, Harz H, Hegemann P (February 1996). "The nature of rhodopsin-triggered photocurrents in Chlamydomonas. I. Kinetics and influence of divalent ions". Biophys. J. 70 (2): 924–931. doi:10.1016/S0006-3495(96)79635-2. PMC 1224992. PMID 8789109.
- Braun FJ, Hegemann P (March 1999). "Two light-activated conductances in the eye of the green alga Volvox carteri". Biophys. J. 76 (3): 1668–1778. doi:10.1016/S0006-3495(99)77326-1. PMC 1300143. PMID 10049347.
- Kateriya, S. Fuhrmann, M. Hegemann, P.: Direct Submission: Chlamydomonas reinhardtii retinal binding protein (cop4) gene; GenBank accession number AF461397
- Suzuki T, Yamasaki K, Fujita S, Oda K, Iseki M, Yoshida K, Watanabe M, Daiyasu H, Toh H, Asamizu E, Tabata S, Miura K, Fukuzawa H, Nakamura S, Takahashi T (February 2003). "Archaeal-type rhodopsins in Chlamydomonas: model structure and intracellular localization". Biochem. Biophys. Res. Commun. 301 (3): 711–7. doi:10.1016/S0006-291X(02)03079-6. PMID 12565839.
- Berthold P, Tsunoda SP, Ernst OP, Mages W, Gradmann D, Hegemann P (June 2008). "Channelrhodopsin-1 initiates phototaxis and photophobic responses in chlamydomonas by immediate light-induced depolarization". Plant Cell. 20 (6): 1665–1677. doi:10.1105/tpc.108.057919. PMC 2483371. PMID 18552201.
- Zemelman BV, Lee GA, Ng M, Miesenböck G (January 2002). "Selective photostimulation of genetically chARGed neurons". Neuron. 33 (1): 15–22. doi:10.1016/S0896-6273(01)00574-8. PMID 11779476.
- Li X, Gutierrez DV, Hanson MG, Han J, Mark MD, Chiel H, Hegemann P, Landmesser LT, Herlitze S (December 2005). "Fast noninvasive activation and inhibition of neural and network activity by vertebrate rhodopsin and green algae channelrhodopsin". Proc. Natl. Acad. Sci. U.S.A. 102 (49): 17816–21. doi:10.1073/pnas.0509030102. PMC 1292990. PMID 16306259.
- Nagel G, Brauner M, Liewald JF, Adeishvili N, Bamberg E, Gottschalk A (December 2005). "Light activation of channelrhodopsin-2 in excitable cells of Caenorhabditis elegans triggers rapid behavioral responses". Curr. Biol. 15 (24): 2279–84. doi:10.1016/j.cub.2005.11.032. PMID 16360690.
- Lima SQ, Miesenböck G (April 2005). "Remote control of behavior through genetically targeted photostimulation of neurons". Cell. 121 (1): 141–52. doi:10.1016/j.cell.2005.02.004. PMID 15820685.
- Zhang F, Wang LP, Boyden ES, Deisseroth K (October 2006). "Channelrhodopsin-2 and optical control of excitable cells". Nat. Methods. 3 (10): 785–92. doi:10.1038/nmeth936. PMID 16990810.
- Lin JY, Lin MZ, Steinbach P, Tsien RY (March 2009). "Characterization of engineered channelrhodopsin variants with further improved photocurrents and kinetics". Biophys. J. 96 (5): 1803–14. doi:10.1016/j.bpj.2008.11.034. PMC 2717302. PMID 19254539.
- Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, de Lecea L (November 2007). "Neural substrates of awakening probed with optogenetic control of hypocretin neurons". Nature. 450 (7168): 420–4. doi:10.1038/nature06310. PMC 6744371. PMID 17943086.
- Tsai HC, Zhang F, Adamantidis A, Stuber GD, Bonci A, de Lecea L, Deisseroth K (May 2009). "Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning". Science. 324 (5930): 1080–4. doi:10.1126/science.1168878. PMC 5262197. PMID 19389999.
- Gradinaru V, Mogri M, Thompson KR, Henderson JM, Deisseroth K (April 2009). "Optical deconstruction of parkinsonian neural circuitry". Science. 324 (5925): 354–9. CiteSeerX 10.1.1.368.668. doi:10.1126/science.1167093. PMC 6744370. PMID 19299587.
- Kravitz AV, Freeze BS, Parker PR, Kay K, Thwin MT, Deisseroth K, Kreitzer AC (July 2010). "Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry". Nature. 466 (7306): 622–6. doi:10.1038/nature09159. PMC 3552484. PMID 20613723.
- Lee JH, Durand R, Gradinaru V, Zhang F, Goshen I, Kim DS, Fenno LE, Ramakrishnan C, Deisseroth K (June 2010). "Global and local fMRI signals driven by neurons defined optogenetically by type and wiring". Nature. 465 (7299): 788–92. doi:10.1038/nature09108. PMC 3177305. PMID 20473285.
- Kätzel D, Zemelman BV, Buetfering C, Wölfel M, Miesenböck G (January 2011). "The columnar and laminar organization of inhibitory connections to neocortical excitatory cells". Nat. Neurosci. 14 (1): 100–7. doi:10.1038/nn.2687. PMC 3011044. PMID 21076426.
- Wang H, Peca J, Matsuzaki M, Matsuzaki K, Noguchi J, Qiu L, Wang D, Zhang F, Boyden E, Deisseroth K, Kasai H, Hall WC, Feng G, Augustine GJ (May 2007). "High-speed mapping of synaptic connectivity using photostimulation in Channelrhodopsin-2 transgenic mice". Proc. Natl. Acad. Sci. U.S.A. 104 (19): 8143–8. doi:10.1073/pnas.0700384104. PMC 1876585. PMID 17483470.
- Mohanty SK, Reinscheid RK, Liu X, Okamura N, Krasieva TB, Berns MW (October 2008). "In-depth activation of channelrhodopsin 2-sensitized excitable cells with high spatial resolution using two-photon excitation with a near-infrared laser microbeam". Biophys. J. 95 (8): 3916–26. doi:10.1529/biophysj.108.130187. PMC 2553121. PMID 18621808.
- Rickgauer JP, Tank DW (September 2009). "Two-photon excitation of channelrhodopsin-2 at saturation". Proc. Natl. Acad. Sci. U.S.A. 106 (35): 15025–30. doi:10.1073/pnas.0907084106. PMC 2736443. PMID 19706471.
- Andrasfalvy BK, Zemelman BV, Tang J, Vaziri A (June 2010). "Two-photon single-cell optogenetic control of neuronal activity by sculpted light". Proc. Natl. Acad. Sci. U.S.A. 107 (26): 11981–6. doi:10.1073/pnas.1006620107. PMC 2900666. PMID 20543137.
- Reiner A, Isacoff EY (October 2013). "The Brain Prize 2013: the optogenetics revolution". Trends in Neurosciences. 36 (10): 557–60. doi:10.1016/j.tins.2013.08.005. PMID 24054067.
Further reading
- Hegemann P. (2008). "Algal sensory photoreceptors". Annu Rev Plant Biol. 59: 167–189. doi:10.1146/annurev.arplant.59.032607.092847. PMID 18444900. (Naturel function of channelrhodopsins and other photoreceptors in green)
- Arenkiel BR, Peca J, Davison IG, et al. (April 2007). "In vivo light-induced activation of neural circuitry in transgenic mice expressing channelrhodopsin-2". Neuron. 54 (2): 205–18. doi:10.1016/j.neuron.2007.03.005. PMC 3634585. PMID 17442243. (Using channelrhodopsin in transgenic mice to study brain circuitry)
- Bi A, Cui J, Ma YP, et al. (April 2006). "Ectopic Expression of a Microbial-Type Rhodopsin Restores Visual Responses in Mice with Photoreceptor Degeneration". Neuron. 50 (1): 23–33. doi:10.1016/j.neuron.2006.02.026. PMC 1459045. PMID 16600853. (Using channelrhodopsin potentially to treat blindness)
External links
- OpenOptogenetics.org, a comprehensive wiki about optogenetics.
- Optogenetics Resource Center / Deisseroth lab
- Boyden lab
- Lab of Zhuo-Hua Pan
- Hegemann lab
- The Brain Prize 2013 for the invention of optogenetics