Carboxypeptidase
A carboxypeptidase (EC number 3.4.16 - 3.4.18) is a protease enzyme that hydrolyzes (cleaves) a peptide bond at the carboxy-terminal (C-terminal) end of a protein or peptide. This is in contrast to an aminopeptidases, which cleave peptide bonds at the N-terminus of proteins. Humans, animals, bacteria and plants contain several types of carboxypeptidases that have diverse functions ranging from catabolism to protein maturation.

Mechanism
The mechanism to produce carboxypeptidase involve that the substrate coordinate water is replaced by substrate of carbonyl (C=O) groups.
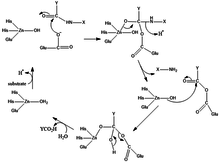
Functions
The first carboxypeptidases studied were those involved in the digestion of food (pancreatic carboxypeptidases A1, A2, and B). However, most of the known carboxypeptidases are not involved in catabolism; they help to mature proteins (e.g., Post-translational modification) or regulate biological processes. For example, the biosynthesis of neuroendocrine peptides such as insulin requires a carboxypeptidase. Carboxypeptidases also function in blood clotting, growth factor production, wound healing, reproduction, and many other processes.
Classifications
By active site mechanism
Carboxypeptidases are usually classified into one of several families based on their active site mechanism.
- Enzymes that use a metal in the active site are called "metallo-carboxypeptidases" (EC number 3.4.17).
- Other carboxypeptidases that use active site serine residues are called "serine carboxypeptidases" (EC number 3.4.16).
- Those that use an active site cysteine are called "cysteine carboxypeptidase" (or "thiol carboxypeptidases")(EC number 3.4.18).
These names do not refer to the selectivity of the amino acid that is cleaved.
By substrate preference
Another classification system for carboxypeptidases refers to their substrate preference.
- In this classification system, carboxypeptidases that have a stronger preference for those amino acids containing aromatic or branched hydrocarbon chains are called carboxypeptidase A (A for aromatic/aliphatic).
- Carboxypeptidases that cleave positively charged amino acids (arginine, lysine) are called carboxypeptidase B (B for basic).
A metallo-carboxypeptidase that cleaves a C-terminal glutamate from the peptide N-acetyl-L-aspartyl-L-glutamate is called "glutamate carboxypeptidase".
A serine carboxypeptidase that cleaves the C-terminal residue from peptides containing the sequence -Pro-Xaa (Pro is proline, Xaa is any amino acid on the C-terminus of a peptide) is called "prolyl carboxypeptidase".
Activation
Some, but not all, carboxypeptidases are initially produced in an inactive form; this precursor form is referred to as a procarboxypeptidase. In the case of pancreatic carboxypeptidase A, the inactive zymogen form - pro-carboxypeptidase A - is converted to its active form - carboxypeptidase A - by the enzyme trypsin. This mechanism ensures that the cells wherein pro-carboxypeptidase A is produced are not themselves digested.
See also
- Carboxypeptidase E
- Carboxypeptidase A
- Enzyme category EC number 3.4
- Thrombin-activatable fibrinolysis inhibitor aka plasma carboxypeptidase B2
- bacterial transpeptidase, an alanine carboxypeptidase
- bradykinin is broken down among other enzymes by carboxypeptidase N
- D-Ala carboxypeptidase is a penicillin-binding protein
- Phenylalanine might inhibit carboxypeptidase A
- Martha L. Ludwig[1]
External links
- Carboxypeptidases at the US National Library of Medicine Medical Subject Headings (MeSH)
- Protease section Stryer book '02