Brody myopathy
Brody myopathy, is a rare disorder that affects skeletal muscle function.[1] BD was first characterized in 1969 by Dr. Irwin A. Brody at Duke University Medical Center.[2] Individuals with BD have difficulty relaxing their muscles after exercise.[2] This difficulty in relaxation leads to symptoms including cramps, stiffness, and discomfort in the muscles of the limbs and face.[2] Symptoms are heightened by exercise and commonly progress in severity throughout adulthood.[1]
| Brody myopathy | |
|---|---|
| Other names | Brody disease (BD) |
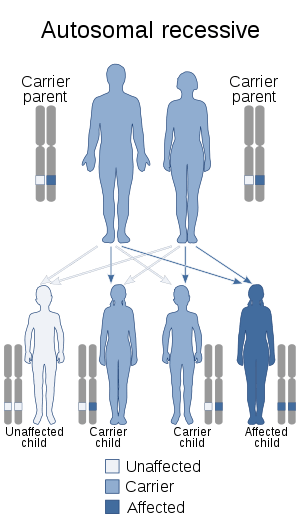 | |
| This condition is inherited in an autosomal recessive manner | |
| Specialty | Neurology |
Cause
Most cases of BD are inherited through autosomal recessive mutations in ATP2A1, where each copy of the affected individual's gene contain a mutation.[1] The gene involved in BD encodes the fast-twitch skeletal muscle ATPase, SERCA1.[3] SERCA1 is a protein pump that uses ATP to pump Ca2+ ions from the cytosol to the sarcoplasmic reticulum in skeletal muscle.[3] In those with BD, SERCA1 pumps are unable to effectively move Ca2+ across the membrane, leading to increased levels of cytoplasmic Ca2+.[3] Increases in cytoplasmic Ca2+ levels interfere with muscle contraction, leading to the characteristic symptoms of BD.[3]
In some cases of BD, no mutations in ATP2A1 have been observed.[1] Disease transmission in cases of non-ATP2A1 BD have been characterized as autosomal dominant pattern of inheritance.[1] These cases have revealed that the cause of the disease likely exhibits genetic heterogeneity, meaning the disease involves mutations in other locations within the genome (although no other loci have been identified in the development of BD as of now).[4]
Diagnosis
Diagnosis of BD begins with clinical evaluation of individuals for characteristic symptoms of cramping and stiffness of exercised muscles.[2] A few techniques are involved in confirming a diagnosis of BD.
Blood testing may be used to measure serum creatine kinase, which ranges from normal to slightly elevated in those with BD.[5] Skeletal muscle biopsies are used to examine muscle fibers. Biopsies in individuals with BD often show variation in muscle fiber size, atrophied fast-twitch muscle fibers, and increased nuclei number.[6] Electromyography (EMG) can be used in diagnosis to rule out myotonia, or muscle stiffness that is detected by EMG. Individuals with BD have stiff muscles but normal EMG results (pseudo-myotonia), where no myotonic discharges are detected.[1] Genetic testing may also be used in the diagnosis of BD to look for mutations in ATP2A1.[7] Since only some forms of the disease are associated with ATP2A1, results of genetic testing do not always confirm a diagnosis of BD, but are useful to rule out other similar disorders.
Treatment
There is no cure for BD, although treatment options are available for reducing the negative symptoms of BD. The drugs dantrolene and verapamil are used in BD treatment due to their effects on Ca2+.[1] Dantrolene is a muscle relaxer that decreases the symptoms of BD by inhibiting Ca2+ release channels in the sarcoplasmic reticulum, while verapamil sequesters Ca2+ in the sarcoplasmic reticulum of muscle cells by functioning as a Ca2+ channel blocker.[8][9]
These drugs act by limiting the amount of Ca2+ from being released from the sarcoplasmic reticulum. When a muscle is stimulated, Ca2+ is released from the sarcoplasmic reticulum into the cytoplasm where it binds to a protein called troponin.[10] This event allows the muscle fibers to overlap, causing a contraction.[10] In BD, Ca2+ levels are high in the cytoplasm, which means Ca2+ can readily bind troponin, leading to muscles that are in an extended state of contraction.[3]
References
- "Brody myopathy | Genetic and Rare Diseases Information Center (GARD) – an NCATS Program". rarediseases.info.nih.gov. Retrieved 2018-09-07.
- Brody IA (July 1969). "Muscle contracture induced by exercise. A syndrome attributable to decreased relaxing factor". The New England Journal of Medicine. 281 (4): 187–92. doi:10.1056/NEJM196907242810403. PMID 4239835.
- Reference, Genetics Home. "ATP2A1 gene". Genetics Home Reference. Retrieved 2018-09-07.
- McKusick, Victor; O'Neill, Marla (2017-12-27). "OMIM Entry - # 601003 - BRODY MYOPATHY". www.omim.org. Retrieved 2018-09-25.
- Poels PJ, Wevers RA, Braakhekke JP, Benders AA, Veerkamp JH, Joosten EM (July 1993). "Exertional rhabdomyolysis in a patient with calcium adenosine triphosphatase deficiency". Journal of Neurology, Neurosurgery, and Psychiatry. 56 (7): 823–6. doi:10.1136/jnnp.56.7.823. PMC 1015068. PMID 8331362.
- Taylor DJ, Brosnan MJ, Arnold DL, Bore PJ, Styles P, Walton J, Radda GK (November 1988). "Ca2+-ATPase deficiency in a patient with an exertional muscle pain syndrome". Journal of Neurology, Neurosurgery, and Psychiatry. 51 (11): 1425–33. doi:10.1136/jnnp.51.11.1425. PMC 1032814. PMID 2976810.
- "Brody myopathy - Conditions - GTR - NCBI". www.ncbi.nlm.nih.gov. Retrieved 2018-09-25.
- "CALAN - verapamil hydrochloride tablet, film coated" (PDF). FDA. 2009.
- Krause T, Gerbershagen MU, Fiege M, Weisshorn R, Wappler F (April 2004). "Dantrolene--a review of its pharmacology, therapeutic use and new developments". Anaesthesia. 59 (4): 364–73. doi:10.1111/j.1365-2044.2004.03658.x. PMID 15023108.
- "Sliding Filament Theory, Sarcomere, Muscle Contraction, Myosin | Learn Science at Scitable". www.nature.com. Retrieved 2018-09-25.
External links
| Classification | |
|---|---|
| External resources |