Bisphenol AF
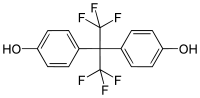
Bisphenol AF (BPAF) is a fluorinated organic compound related to bisphenol A in which the two methyl groups are replaced with trifluoromethyl groups.
 | |
| Names | |
|---|---|
| IUPAC name
4-[1,1,1,3,3,3-Hexafluoro-2-(4-hydroxyphenyl)propan-2-yl]phenol | |
| Other names
Biphenol AF; Hexafluorobisphenol A; Hexafluorodiphenylolpropane; Bisphenol A hexafluoride; 4,4'-(Hexafluoroisopropylidene)diphenol; Hexafluoroacetone bisphenol A; 2,2-Bis(4-hydroxyphenyl)hexafluoropropane | |
| Identifiers | |
3D model (JSmol) |
|
| Abbreviations | BPAF |
| ChemSpider | |
| ECHA InfoCard | 100.014.579 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C15H10F6O2 | |
| Molar mass | 336.233 g·mol−1 |
| Melting point | 162 °C[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Whereas BPA binds with human estrogen-related receptor gamma (ERR-γ), BPAF all but ignores ERR-γ. Instead, BPAF activates ERR-α and binds to and disables ERR-β.[2]
See also
References
- Hayasaka, Tatsuya; Katsuhara, Yutaka; Kume, Takashi; Yamazaki, Takashi (2011). "HF-mediated equilibrium between fluorinated ketones and the corresponding α-fluoroalcohols". Tetrahedron. 67 (12): 2215–2219. doi:10.1016/j.tet.2011.01.087.
- Janet Raloff: Another plastics ingredient raises safety concerns, Science News, June 5th, 2010; Vol.177 #12 (p. 14)
- Hayasaka, Tatsuya; Katsuhara, Yutaka; Kume, Takashi; Yamazaki, Takashi (2011). "HF-mediated equilibrium between fluorinated ketones and the corresponding α-fluoroalcohols". Tetrahedron. 67 (12): 2215–2219. doi:10.1016/j.tet.2011.01.087.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.