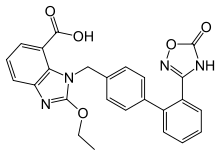
Azilsartan
Azilsartan is an angiotensin II receptor antagonist used in the treatment of hypertension,[2] developed by Takeda. It is marketed in tablet form under the brand name Edarbi as the prodrug azilsartan medoxomil.[3]
 | |
| Clinical data | |
|---|---|
| Trade names | Edarbi, Azilva |
| Other names | TAK-536, TAK-491 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a611028 |
| License data |
|
| Pregnancy category | |
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 60% |
| Metabolism | CYP2C9 |
| Elimination half-life | 11 hrs |
| Excretion | 55% feces, 42% urine |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.235.975 |
| Chemical and physical data | |
| Formula | C25H20N4O5 |
| Molar mass | 456.458 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
The most common adverse reaction in adults is diarrhea.[2]
It is also sold as a combination drug with chlortalidone under the brand name Edarbyclor.[4]
Medical uses
Azilsartan is used for the treatment of hypertension in adults.[3][5][2]
Contraindications
Azilsartan must not be used with aliskiren, a renin inhibitor, in patients with diabetes as this increases the risk of serious adverse effects.[3][2] Like other antihypertensive drugs acting on the renin–angiotensin system, it is contraindicated during the second and third trimesters of pregnancy.[3][5][6] It should not be used during pregnancy in the United States.[2][1]
Interactions
No relevant drug interactions have been found in studies. Based on experiences with other drugs acting on the renin–angiotensin system, it is theorized that azilsartan could increase the toxicity of lithium and of other drugs increasing potassium levels, such as potassium sparing diuretics.[5][6]
Pharmacology
Mechanism of action
Azilsartan medoxomil lowers blood pressure by blocking the action of angiotensin II at the AT1 receptor, a hormone that contracts blood vessels and reduces water excretion through the kidneys.[5]
Pharmacokinetics
Azilsartan medoxomil is quickly absorbed from the gut, independently of food intake. Maximal blood plasma concentrations are reached after one to three hours. The liver enzyme CYP2C9 is involved in the formation of the two main metabolites, which are pharmacologically inactive; they are the O-deethylation and decarboxylation products of azilsartan. Elimination half life is about 11 hours. 55% are excreted via the feces, and 42% via the urine, of which 15% are present as azilsartan and the rest in form of the metabolites.[6]
Chemistry

The drug formulation contains the potassium salt of azilsartan medoxomil (codenamed TAK-491), an ester of azilsartan's carboxyl group with the alcohol (5-methyl-2-oxo-1,3-dioxol-4-yl)methanol.[6] This ester is more lipophilic than azilsartan itself.
History
In February 2011, the U.S. Food and Drug Administration (FDA) approved azilsartan medoxomil for the treatment of high blood pressure in adults.[7][8] In July 2011, azilsartan medoxomil was approved in the European Union for the treatment of essential hypertension.[3] In March 2012, Health Canada approved the drug for mild to moderate essential hypertension.[9]
In December 2014, Valeant Canada acquired the marketing rights to Edarbi and Edarbyclor from Takeda Pharmaceutical."Valeant Canada acquires rights to Edarbi and Edarbyclor for the Canadian market" (Press release). Valeant Canada. 17 December 2014. Retrieved 9 March 2020 – via Cision.</ref>
References
- "Azilsartan medoxomil (Edarbi) Use During Pregnancy". Drugs.com. 28 February 2020. Retrieved 9 March 2020.
- "Edarbi- azilsartan kamedoxomil tablet". DailyMed. 26 July 2019. Retrieved 9 March 2020.
- "Edarbi EPAR". European Medicines Agency (EMA). 18 May 2018. Retrieved 9 March 2020.
- "Drug Approval Package:Edarbyclor (azilsartan medoxomil and chlorthalidone) NDA #202331". U.S. Food and Drug Administration (FDA). 16 August 2012. Retrieved 11 March 2020.
- Haberfeld, H, ed. (2015). Austria-Codex (in German). Vienna: Österreichischer Apothekerverlag. Edarbi-Tabletten.
- Dinnendahl, V; Fricke, U, eds. (2012). Arzneistoff-Profile (in German). 2 (26 ed.). Eschborn, Germany: Govi Pharmazeutischer Verlag. ISBN 978-3-7741-9846-3.
- "Drug Approval Package: Edarbi (Azilsartan medoxomil) NDA 200796". U.S. Food and Drug Administration (FDA). 4 April 2011. Retrieved 9 March 2020.
- "FDA approves Edarbi to treat high blood pressure" (Press release). U.S. Food and Drug Administration. 25 February 2011. Archived from the original on 18 January 2017. Retrieved 1 March 2011.
- Notice of Decision for Edarbi
External links
- "Azilsartan". Drug Information Portal. U.S. National Library of Medicine.
- "Azilsartan medoxomil". Drug Information Portal. U.S. National Library of Medicine.