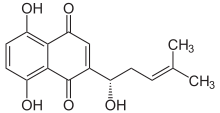
Alkannin
Alkannin is a natural dye that is obtained from the extracts of plants from the borage family Alkanna tinctoria that are found in the south of France. The dye is used as a food coloring and in cosmetics. It is used as a red-brown food additive in regions such as Australia.[2]. Alkannin has a deep red color in a greasy or oily environment and a violet color in an alkaline environment.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
5,8-Dihydroxy-2-[(1S)-1-hydroxy-4-methylpent-3-en-1-yl]naphthalene-1,4-dione | |
| Other names
C.I. Natural red 20 Alkanet extract Anchusaic acid Anchusin | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.007.497 |
| E number | E103 (colours) |
| KEGG | |
PubChem CID |
|
| UNII | |
| |
| |
| Properties[1] | |
| C16H16O5 | |
| Molar mass | 288.299 g·mol−1 |
| Appearance | Red-brown crystalline prisms |
| Density | 1.15 g/mL |
| Melting point | 149 °C (300 °F; 422 K) |
| Boiling point | 567 °C (1,053 °F; 840 K) |
| Sparingly soluble | |
| Hazards | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
3.0 g/kg (mice) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
The chemical structure as a naphthoquinone derivative was first determined by Brockmann in 1936.[3] The enantiomer of alkannin is known as shikonin, and the racemic mixture of the two is known as shikalkin.[4][5]
The enzyme 4-hydroxybenzoate geranyltransferase utilizes geranyl diphosphate and 4-hydroxybenzoate to produce 3-geranyl-4-hydroxybenzoate and diphosphate. These compounds are then used to form alkannin.[5]
Alkannin is an antioxidant[6] and has an antimicrobial effect against Staphylococcus aureus and Staphylococcus epidermidis. It is also known to have wound healing, antitumor, and antithrombotic properties.[5]
Shikonin is also found in the Chinese herbal medicine plant Lithospermum erythrorhizon, the red-root gromwell, (紫草 zicao, Pinyin: zǐcǎo).
Shikonin is a plant secondary metabolite that has attracted high research attention due to their notable pharmacological activities, which include antimicrobial, anticancer, wound healing, anti-inflammatory, and antithrombotic uses.[8] Shikonin and its derivatives are the napthoquinone pigments, distributed among members of the family Boraginaceae. These include different species of Lithospermum, Arnebia, Alkanna, Anchusa, Echium and Onosma. [9] Because the root bark (cork layers) of this plant contains large amounts of red naphthoquinone pigments, the roots of these plants are red-purple in color. If shikonin is extracted from fresh tissues, it gradually darkens over several days, finally becoming black precipitates, which are thought to be polymers. [10]
Shikonine has different anticancer effects. this material can inhibit the activities of DNA topoisomerases, which plays a crucial role in cancer cell DNA regulation, including replication, recombination, and transcription. Other mechanisms of shikonin-induced cancer cell death include increased expression of p53 and inhibition of cancer cell glycolysis via targeting pyruvate kinase M2 (PKM2). Since cancer cells rely heavily on glycolysis and PKM2 is the last rate-limiting enzyme in glycolysis, shikonin, a naturally occurred PKM2 inhibitor, might be able to suppress cancer cell growth by inhibiting the main source of ATP production. In addition, shikonin’s effect on cellular metabolism may also render its activity to sensitize resistant cells to chemotherapy.[11] shikonin cytotoxic actions on cancer cells are largely through enhancing reactive oxygen species (ROS) generation to trigger caspase-dependent apoptosis and to downregulate nuclear factor kappa B- (NFkB-) mediated matrix metalloproteinase (MMP) expressions to reduce tumor survival and invasion. It has also been suggested as an adjuvant to treat lung cancer cells. shikonine induces apoptosis, necrosis and Senescence of cancer cells through increased expression of P53 tumor suppressor gene. Experiments have also been shown that the levels of p53, p16, and cleaved caspase-3 significantly increased at 24 hours after the induction of shikonin (doses at 1.0 and 2.5 𝜇g/mL); at this time, the release of cytochrome 𝑐 from mitochondria to cytosol was also observed.[12]
References
- The Merck Index, 11th Edition, 243
- Additives Archived 2011-04-06 at the Wayback Machine, Food Standards Australia New Zealand
- H. Brockmann (1936). "Die Konstitution des Alkannins, Shikonins und Alkannans". Justus Liebigs Ann. Chem. 521: 1–47. doi:10.1002/jlac.19365210102.
- Shmuel Yannai (2012). Dictionary of Food Compounds. CRC Press. p. 478.
- Vassilios P. Papageorgiou; Andreana N. Assimopoulou; Elias A. Couladouros; David Hepworth; K. C. Nicolaou (1999). "The Chemistry and Biology of Alkannin, Shikonin, and Related Naphthazarin Natural Products". Angew. Chem. Int. Ed. 38 (3): 270–300. doi:10.1002/(SICI)1521-3773(19990201)38:3<270::AID-ANIE270>3.0.CO;2-0.
- A.N. Assimopoulou; D. Boskou; V.P. Papageorgiou (2004). "Antioxidant activities of alkannin, shikonin and Alkanna tinctoria root extracts in oil substrates". Food Chemistry. 87 (3): 433–438. doi:10.1016/j.foodchem.2003.12.017.
- Wiench, B; Eichhorn, T; Paulsen, M; Efferth, T (2012). "Shikonin Directly Targets Mitochondria and Causes Mitochondrial Dysfunction in Cancer Cells". Evidence-Based Complementary and Alternative Medicine. 2012: 726025. doi:10.1155/2012/726025. PMC 3478753. PMID 23118796.
- Sut S. , Pavela R. (2017). Identification of Onosma visianii Roots Extract and Purified Shikonin Derivatives as Potential Acaricidal Agents against Tetranychus urticae. Molecules.
- Malic S. , Bhushan Sh. , Sharma M and ahuja PS. (2016). Biotechnological approaches to the production of shikonins: a critical review with recent updates. Crit Rev Biotechnol. 36:327-40.
- Kazufumi Y. (2017). Lithospermum erythrorhizon cell cultures: Present and future aspects. Plant Biotechnology 34: 131–142.
- Li W. , Liu J. , Jackson K. , Shi R. , Zhao Y. (2014). Sensitizing the therapeutic efficacy of taxol with shikonin in human breast cancer cells. PloS one. 9: e94079.
- Yeh Y.C. , Liu T.J. , Lai H.C. (2015). Shikonin induces apoptosis, necrosis, and premature senescence of human A549 lung cancer cells through Upregulation of p53 Expression. Evid Based Complement Alternat Med 2015: 620383