Adrenocortical adenoma
Adrenocortical adenoma is commonly described as a benign neoplasm emerging from the cells that comprise the adrenal cortex. Like most adenomas, the adrenocortical adenoma is considered a benign tumor since the majority of them are non-functioning and asymptomatic. Adrenocortical adenomas are classified as ACTH-independent disorders, and are commonly associated with conditions linked to hyperadrenalism such as Cushing's syndrome (hypercortisolism) or Conn's syndrome (hyperaldosteronism), which is also known as primary aldosteronism.[1] In addition, recent case reports further support the affiliation of adrenocortical adenomas with hyperandrogenism or florid hyperandrogenism which can cause hyperandrogenic hirsutism in females.[2] "Cushing's syndrome" differs from the "Cushing's disease" even though both conditions are induced by hypercortisolism. The term "Cushing's disease" refers specifically to "secondary hypercortisolism" classified as "ACTH-dependent Cushing's syndrome" caused by pituitary adenomas. In contrast, "Cushing's syndrome" refers specifically to "primary hypercortisolism" classified as "ACTH-independent Cushing's syndrome" caused by adrenal adenomas.
| Adrenocortical Adenoma | |
|---|---|
| Other names | Adrenal cortical adenoma, adrenal adenoma |
 | |
| Adrenal adenoma in a patient with Conn syndrome | |
| Specialty | Endocrinology, oncology |
Presentation
| Hypercortisolism | |
|---|---|
 | |
| Specialty | Endocrinology, oncology |
| Symptoms | Adrenal Lesion |
Adrenal adenomas are often categorized as endocrine-inactive tumors considering that majority of them are non-functioning and asymptomatic. Functional adrenocortical adenomas demonstrate symptoms consistent with mixed endocrine syndromes. In most reported cases of adrenocortical adenoma, patients have presented with one or multiple endocrine syndromes such as hyperaldosteronism/Conn's Syndrome,[3] hypercortisolism/Cushing's syndrome,[4] hyperandrogenism/feminization,[5] virilization,[6] or hirsutism.[7] Some of the common symptoms associated with adrenocortical adenomas include:
Musculoskeletal
Cardiovascular
Endocrine and Metabolic
→More prevalent in males
→More prevalent in females
- Hyperandrogenism
- Irregular menstrual cycles
Neuropsychological
- Sleep disorders
- Depression
Skin
- Easy bruising
- Stretch marks
- Hirsutism
- Acne
Cause
| Adrenal Glands Zonations | |
|---|---|
 | |
| Specialty | Endocrinology, oncology |
| Adrenal Glands Zonations | |
|---|---|
 | |
| Specialty | Endocrinology, oncology |
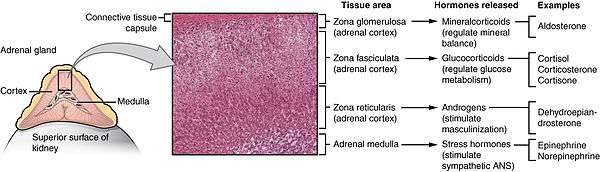
Study of the reported cases indicate that most adrenocortical adenomas occur due to neoplastic proliferation of adrenal cortical cells within the three distinct layers of adrenal cortex. In humans, the adrenal cortex comprises three concentric zones including the zona glomerulosa, zona fasciculata, and zona reticularis that under normal conditions respond to body's physiological demands for steroid hormones. The adrenal cortex is considered a dynamic organ in which senescent cells are replaced by newly differentiated cells. This constant renewal facilitates organ remodeling which contributes to dynamic characteristics of the adrenal cortex. [8] correspondingly, the developmental physiology of the adrenal cortex is believed to play a pivotal role in formation of the adrenocortical tumors. Hence, the molecular mechanisms involved in normal development of the adrenal glands are like double edged swords that can lead to the formation of tumors within the adrenal cortex. Moreover, recent studies suggest that mutations affecting the molecular pathways of the adrenocortical region can stimulate abnormal proliferation and tumor formation. Through these studies, the cyclic AMP-dependent protein kinase A signaling has been identified as a key mediator of cortisol secretion, and the mutations associated with the dysregulation of cyclic AMP - protein kinase A pathways have been implicated in the adrenocortical pathophysiology.[9]
Pathophysiology
If functional, adrenocortical adenomas can affect the normal activities of the adrenal cortex. Located within the adrenal glands are the three zones that are responsible for secretion of the three major classes of adrenal steroids. Hence, functional adrenocortical adenomas can induce over-secretion of adrenal steroids associated with pure or mixed endocrine syndromes, a condition commonly known as hyperadrenalism.
Diagnosis
Due to their asymptomatic nature, most reported cases of adrenal adenomas have been discerned fortuitously through autopsy, or during medical imaging, particularly CT scan (computed tomography) and magnetic resonance imaging. Hence, they have earned the title incidentaloma referring to small adenoma discovered incidentally.[10] Though adrenocortical adenomas are considered challenging to differentiate from the normal adrenal cortex, they appear as well-circumscribed lesions once isolated.
Imaging Diagnostics
- Computed Tomography (CT scan)
- Magnetic Resonance Imaging (MRI)
Laboratory Tests
- CRH Stimulation Test
- High-dose-dexamethasone suppression test
Gross Description
- Well-circumscribed lesion
- Size ≤ 5 cm
- Weight ≤ 50 grams
- often appear as golden-yellow color mass
(may have focal dark regions corresponding to hemorrhage, lipid-depletion, and increased lipofuscin)[10]
Histopathology
The microscopic histopathology analysis of the tissue samples obtained from the adrenal cortex of individuals presenting with adenoma-associated symptoms such as primary aldestronism (PA) indicates that adenoma cells are relatively larger with different cytoplasm, and increased variation in nuclear size. This indication is based on comparison between the healthy (normal) and affected (adenoma-associated) adrenal cortex tissue samples.
Treatment
- Non-functioning cases of adrenocortical adenoma can be managed through long-term followups and monitoring.
- The treatment approach for the functioning cases of adrenocortical adenoma depends on the type of disorders they induce and their advancement. Surgical excision may be required if its presence is resulting in atrophy of the adrenal glands and the surrounding tissues.
In order to acquire better treatment strategies, it is important to further examine, study and discern the distinct molecular mechanisms involved in the formation of endogenous Adrenal Adenomas, hyperplasias, and ACTH-independent Cushing's Syndrome to improve the available diagnostic and prognostic markers that can assist clinicians in the management and advance-treatment of such conditions.[11]
Prognosis
- The long-term outlook for individuals diagnosed with non-functional adrenocortical adenoma is usually excellent.
- The long-term outlook for individuals diagnosed with functional adrenocortical adenoma is good with early diagnosis and treatment.
Epidemiology
- Prevalence: Female > Male
- More common in adults
- Relatively earlier onset in females (ages ≤ 20) than males (ages ≤ 30)
- Most common cause of ACTH-independent Cushing's syndrome
See also
- Hyperplasia
- Adrenal tumor
- Cushing's syndrome
- Conn's syndrome
- Hypercortisolism
- Hyperaldosteronism
- Hyperandrogenism
- Adrenal gland
- Adrenal paraganglioma
- Adrenal Pheochromocytoma
- Adrenal ganglioneuroma
References
- "Definition: adrenocortical adenoma from Online Medical Dictionary".
- LaVoie, Melanie; Constantinides, Vasilis; Robin, Noel; Kyriacou, Angelos (30 July 2018). "Florid hyperandrogenism due to a benign adrenocortical adenoma". BMJ Case Reports. 2018: bcr-2018-224804. doi:10.1136/bcr-2018-224804. PMID 30061126.
- Wang, Wei; Wei, Feng; Li, RanHao; Tian, JiaHui (October 2019). "A case report of idiopathic hyperaldosteronism characterized by bilateral adrenal adenoma". Medicine. 98 (43): e17418. doi:10.1097/MD.0000000000017418. PMC 6824822. PMID 31651844.
- Ren, Kaiyun; Wei, Jia; Liu, Qilin; Zhu, Yuchun; Wu, Nianwei; Tang, Ying; Li, Qianrui; Zhang, Qianying; Yu, Yerong; An, Zhenmei; Chen, Jing; Li, Jianwei (17 June 2019). "Hypercortisolism and primary aldosteronism caused by bilateral adrenocortical adenomas: a case report". BMC Endocrine Disorders. 19: 63. doi:10.1186/s12902-019-0395-y. PMC 6580498. PMID 31208392.
- LaVoie, Melanie; Constantinides, Vasilis; Robin, Noel; Kyriacou, Angelos (30 July 2018). "Florid hyperandrogenism due to a benign adrenocortical adenoma". BMJ Case Reports. 2018: bcr-2018-224804. doi:10.1136/bcr-2018-224804. PMID 30061126.
- Kobayashi, Toshihiro; Imachi, Hitomi; Sato, Seisuke; Ibata, Tomohiro; Fukunaga, Kensaku; Yoshimoto, Takuo; Kikuchi, Fumi; Yonezaki, Kazuko; Yamaji, Nao; Lyu, Jingya; Dong, Tao; Nagata, Hiromi; Kadota, Kyuichi; Kushida, Yoshio; Haba, Reiji; Murao, Koji (1 February 2019). "Bilateral Adrenocortical Adenomas along with Virilization and Cushing's Syndrome". Internal Medicine. 58 (3): 405–409. doi:10.2169/internalmedicine.0790-18. PMC 6395137. PMID 30210105.
- Rodríguez-Gutiérrez, René; Bautista-Medina, Mario Arturo; Teniente-Sanchez, Ana Eugenia; Zapata-Rivera, Maria Azucena; Montes-Villarreal, Juan (2013). "Pure Androgen-Secreting Adrenal Adenoma Associated with Resistant Hypertension". Case Reports in Endocrinology. 2013: 356086. doi:10.1155/2013/356086. PMC 3681270. PMID 23819074.
- Pihlajoki, Marjut; Dörner, Julia; Cochran, Rebecca S.; Heikinheimo, Markku; Wilson, David B. (5 March 2015). "Adrenocortical Zonation, Renewal, and Remodeling". Frontiers in Endocrinology. 6: 27. doi:10.3389/fendo.2015.00027. PMC 4350438. PMID 25798129.
- Lodish, Maya (June 2017). "Genetics of Adrenocortical Development and Tumors". Endocrinology and Metabolism Clinics of North America. 46 (2): 419–433. doi:10.1016/j.ecl.2017.01.007. PMC 5424622. PMID 28476230.
- Lloyd, Ricardo V. (April 2011). "Adrenal cortical tumors, pheochromocytomas and paragangliomas". Modern Pathology. 24: S58–65. doi:10.1038/modpathol.2010.126. PMID 21455202.
- Bourdeau, Isabelle; Lampron, Antoine; Costa, Marcia Helena Soares; Tadjine, Mimi; Lacroix, André (June 2007). "Adrenocorticotropic hormone-independent Cushing's syndrome". Current Opinion in Endocrinology, Diabetes and Obesity. 14 (3): 219–225. doi:10.1097/MED.0b013e32814db842. PMID 17940443.


_adrenocortical_adenoma.jpg)
_adrenocortical_adenoma.jpg)
_adrenocortical_adenoma.jpg)