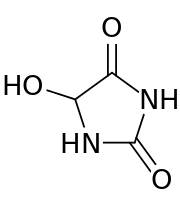
5-Hydroxyhydantoin
5-Hydroxyhydantoin is an oxidation product of 2′-deoxycytidine. If not repaired, it may be processed by DNA polymerases that induce mutagenic processes.[1]
 | |
| Names | |
|---|---|
| IUPAC name
5-Hydroxy-2,4-imidazolidinedione | |
| Other names
Glyoxalurea; Allanturic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C3H4N2O3 | |
| Molar mass | 116.076 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
References
- Gasparutto, Didier; Muller, Evelyne; Boiteux, Serge; Cadet, Jean (January 2009). "Excision of the oxidatively formed 5-hydroxyhydantoin and 5-hydroxy-5-methylhydantoin pyrimidine lesions by Escherichia coli and Saccharomyces cerevisiae DNA N-glycosylases". Biochimica et Biophysica Acta (BBA) - General Subjects. 1790 (1): 16–24. doi:10.1016/j.bbagen.2008.10.001. PMID 18983898.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.