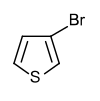
3-Bromothiophene
3-Bromothiophene is an organosulfur compound with the formula C4H3BrS. It is a colorless liquid. It is a precursor to the antibiotic timentin and the vasodilator cetiedil.[1]
 | |
| Names | |
|---|---|
| Preferred IUPAC name
3-Bromothiophene | |
| Other names
3-Thienyl bromide, 3BT | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.011.656 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C4H3BrS | |
| Molar mass | 163.03 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 1.74 g/mL |
| Melting point | −10 °C (14 °F; 263 K) |
| Boiling point | 150–158 °C (302–316 °F; 423–431 K) |
| Immiscible | |
| Hazards | |
| Main hazards | N,Xi,Xn,T |
| GHS pictograms |     |
| GHS Signal word | Danger |
GHS hazard statements |
H226, H301, H310, H310, H315, H317, H319, H330, H335, H411 |
| P210, P233, P240, P241, P242, P243, P260, P261, P262, P264, P270, P271, P272, P273, P280, P284, P301+310, P302+350, P302+352, P303+361+353, P304+340, P305+351+338, P310, P312, P320 | |
| Flash point | 56 °C (133 °F; 329 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Preparation
Unlike 2-bromothiophene, the 3-bromo isomer cannot be prepared directly from thiophene. It can be prepared by debromination of 2,3,5-tribromothiophene,[2] which is obtained by bromination of thiophene.
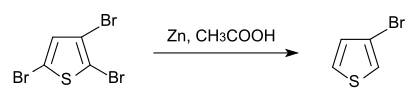
Synthese van 3-broomthiofeen
See also
References
- Jonathan Swanston (2006). "Thiophene". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a26_793.pub2.
- S. Gronowitz (1959). "3-Bromothiophene". Org. Syntheses. 44: 9. doi:10.15227/orgsyn.044.0009.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.