2-Phenethyl propionate
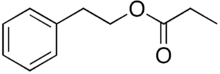
2-Phenethyl propionate, also known as phenethyl propanoate or phenylethyl propionate,[2] is the ester of phenethyl alcohol and propionic acid. It can be found in peanuts.[3]
 | |
| Names | |
|---|---|
| IUPAC name
2-Phenylethyl propanoate | |
| Other names
2-Phenethyl propanoate; Phenylethyl propionate; Phenethyl propanoate | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.004.153 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C11H14O2 | |
| Molar mass | 178.231 g·mol−1 |
| Odor | Floral, rose, sweet[1] |
| Density | 1.007 g/mL[1] |
| Boiling point | 245 °C (473 °F; 518 K)[1] |
| Hazards | |
EU classification (DSD) (outdated) |
Irritant (Xi) |
| Flash point | 113 °C (235 °F; 386 K) |
| Related compounds | |
Related compounds |
Eugenol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
It has shown antifungal activity[4] and was tested as a pesticide.[5] It is used in some preparations used in the management of bed bugs[6] and in other pesticide products.[7] In the U.S it is considered a "minimal risk pesticide" and can be used as a pesticide without any registration.[8]
References
- Phenethyl propionate at Sigma-Aldrich
- CID 31225 from PubChem
- CID 31225 from PubChem
- Dev, U.; Devakumar, C.; Mohan, J.; Agarwal, P.C. (2004). "Antifungal activity of aroma chemicals against seed-borne fungi". Journal of essential oil research. 16 (5): 496–499. doi:10.1080/10412905.2004.9698780. Archived from the original on 7 August 2008. Retrieved 26 April 2008.
- Murray B. Isman (2000). "Plant essential oils for pest and disease management". Crop Protection. 19 (8–10): 603–608. doi:10.1016/S0261-2194(00)00079-X.
- "U.S. EPA bed bug products search results". Archived from the original on 15 October 2010. Retrieved 2 November 2010.
- "Lawn Insect Killer (32 oz hose end)". EcoSmart. Archived from the original on September 23, 2014. Retrieved September 7, 2014.
- Ralf-Udo Ehlers, ed. (2011). Regulation of Biological Control Agents In Europe. ISBN 9789048136643.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.