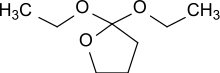
2,2-Diethoxytetrahydrofuran
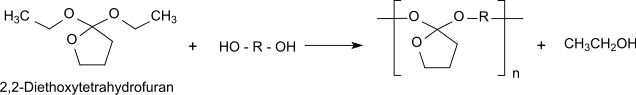
2,2-Diethoxytetrahydrofuran is a cyclic orthoester which can be reacted with diols to biodegradable polyorthoesters.
 | |
| Names | |
|---|---|
| IUPAC name
2,2-Diethoxyoxolane | |
| Identifiers | |
3D model (JSmol) |
|
| ECHA InfoCard | 100.161.490 |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C8H16O3 | |
| Molar mass | 160.213 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Preparation
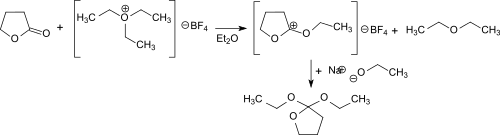
The synthesis of 2,2-diethoxytetrahydrofuran via γ-butyrolactone and the Meerwein salt (triethyloxonium tetrafluoroborate) in diethyl ether was first described by Hans Meerwein and co-workers.[1] In the reaction the electrophilic ethyl cation attackes the carbonyl oxygen and forms the stable but extraordinarily hygroscopic O-ethyl-γ-butyrolactonium tetrafluoroborate (melting point 42 °C). The compound dissolves in dichloromethane, chloroform and 1,2-dichloroethane but is insoluble in diethyl ether, benzene and tetrachloromethane. The onium salt reacts practically quantitatively with an ethanolate anion from sodium ethoxide in ethanol forming 2,2-diethoxytetrahydrofuran.
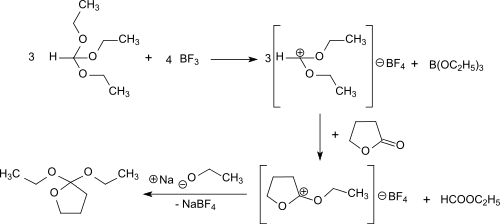
2,2-Diethoxytetrahydrofuran can also be produced in a solvent-free one-pot reaction using γ-butyrolactone, orthoformic triethyl ester and gaseous boron trifluoride. This route avoids the use of diethyl ether and its side-products and sensible intermediates.[2]
First diethoxymethylium tetrafluoroborate is formed from the triethyl orthoformate and boron trifluoride at -30 °C. This electrophilically attacks the carbonyl group of the γ-butyrolactone and the O-ethyl-γ-butyrolactonium tetrafluoroborate. The addition of sodium ethoxide leads to the final product, which is obtained after distillation in 69% overall yield.
The reaction proceeds under gentle conditions (<0 °C) and the almost quantitative addition of ethanolate to O-ethyl-γ-butyrolactonium tetrafluoroborate can also be catalyzed by bases such as ammonia and triethylamine.
Properties
2,2-Diethoxytetrahydrofuran is a clear liquid which boils at 10 mm Hg vacuum at 60 - 61.5 °C according to the original literature.[1]
Application
The cyclic orthoester 2,2-diethoxytetrahydrofuran is a reactive bifunctional monomer which forms biodegradable polyorthoesters of the type POE-I by transesterification with α, ω-diols.
Polyorthoesters are used as embedding media for pharmaceuticals in depot drug dosage forms for controlled drug release by surface erosion under physiological conditions.[3]
References
- H. Meerwein; P. Borner; O. Fuchs; H.J. Sasse; H. Schrodt; J. Spille (1956), "Reaktionen mit Alkylkationen", Chem. Ber. (in German), 89 (9), pp. 2060–2079, doi:10.1002/cber.19560890907
- US 4990631, K. Alster, "Reacting trialkyl orthoformate with boron trifluoride and lactone, then with alkoxide or alkanol and base"
- Jorge Heller (1997), A.J. Domb; J. Kost; D.M. Wiseman (eds.), "6. Poly (Ortho Esters)", Handbook of Biodegradable Polymers, Harwood Academic Press, pp. 99–118, ISBN 90-5702-153-6