1,8-Diaminonaphthalene
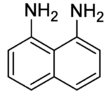
1,8-Diaminonaphthalene is an organic compound with the formula C10H6(NH2)2. It is one of several isomeric naphthalenediamines. It is a colorless solid that darkens in air due to oxidation. It is a precursor to commercial pigments.[1]
 | |
| Names | |
|---|---|
| IUPAC name
naphthalene-1,8-diamine | |
| Other names
deltamin, 1,8-naphthalenediamine | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.006.846 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H10N2 | |
| Molar mass | 158.1998 |
| Related compounds | |
Related Aromatic amines |
1-Naphthylamine 1,8-bis(dimethylamino)naphthalene |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Synthesis and reactions

Chemical structure of 12-phthaloperinone, a derivative of 1,8-diaminonaphthalene.
It is prepared by reduction of 1,8-dinitronaphthalene, which in turn is obtained as a mixture of isomers by nitration of 1-nitronaphthalene.
Upon treatment with phthalic anhydride derivatives, the diamine converts to phthaloperinones.[2] The derivative from phthalic anhydride itself, Solvent Orange 60, is a useful orange pigment. It is a precursor to 1,8-bis(dimethylamino)naphthalene.
See also
References
- Booth, Gerald (2005). "Naphthalene Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH..
- Mamada, Masashi; PéRez-BolíVar, César; Anzenbacher, Pavel (2011). "Green Synthesis of Polycyclic Benzimidazole Derivatives and Organic Semiconductors". Organic Letters. 13 (18): 4882–4885. doi:10.1021/ol201973w. PMID 21863817.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.