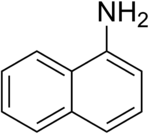
1-Naphthylamine
1-Naphthylamine is an aromatic amine derived from naphthalene. It can cause bladder cancer (transitional cell carcinoma). It crystallizes in colorless needles which melt at 50 °C. It possesses a disagreeable odor, sublimes readily, and turns brown on exposure to air. It is the precursor to a variety of dyes.[2]
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Naphthalen-1-amine | |
| Other names
(Naphthalen-1-yl)amine 1-Naphthylamine α-Naphthylamine 1-Aminonaphthalene | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.004.672 |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H9N | |
| Molar mass | 143.19 g/mol |
| Appearance | Colorless crystals (reddish-purple in air)[1] |
| Odor | ammonia-like[1] |
| Density | 1.114 g/cm3 |
| Melting point | 47 to 50 °C (117 to 122 °F; 320 to 323 K) |
| Boiling point | 301 °C (574 °F; 574 K) |
| 0.002% (20°C)[1] | |
| Vapor pressure | 1 mmHg (104° C)[1] |
| |
| Hazards | |
| Safety data sheet | |
| Flash point | 157 °C; 315 °F; 430 K[1] |
| Related compounds | |
Related compounds |
2-Naphthylamine 1-Naphthol Naphthalene Aniline 1,8-Bis(dimethylamino)naphthalene |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Preparation and reactions
It can be prepared by reducing 1-nitronaphthalene with iron and hydrochloric acid followed by steam distillation.[2]
Oxidizing agents, such as ferric chloride, give a blue precipitate with solutions of its salts. Chromic acid converts it into 1-naphthoquinone. Sodium in boiling amyl alcohol reduces the unsubstituted ring, giving tetrahydro-1-naphthylamine. This tetrahydro compound yields adipic acid when oxidized by potassium permanganate.
At 200 °C in sulfuric acid, it converts to 1-naphthol.
Use in dyes
The sulfonic acid derivatives of 1-naphthylamine are used for the preparation of azo dye. These compounds possess the important property of dyeing unmordanted cotton.
An important derivative is naphthionic acid (1-aminonaphthalene-4-sulfonic acid), which produced by heating 1-naphthylamine and sulfuric acid to 170–180 °C in the presence of crystallized oxalic acid. It forms small needles, very sparingly soluble in water. Upon treatment with the bis(diazonium) derivative of benzidine, 1-aminonaphthalene-4-sulfonic acid gives Congo red.
Safety
It is listed as one of the 13 carcinogens covered by the OSHA General Industry Standards.[3]
See also
- Used in preparation of aptiganel.
References
- NIOSH Pocket Guide to Chemical Hazards. "#0441". National Institute for Occupational Safety and Health (NIOSH).
- Gerald Booth (2005). "Naphthalene Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a17_009..
- OSHA Standard 1910.1003