Xanthohumol
 | |
| Names | |
|---|---|
| IUPAC name
(E)-1-[2,4-Dihydroxy-6-methoxy-3-(3-methylbut-2-enyl)phenyl]-3-(4-hydroxyphenyl)prop-2-en-1-one | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.123.285 |
PubChem CID |
|
| RTECS number | UD5574117 |
| UNII | |
| |
| |
| Properties | |
| C21H22O5 | |
| Molar mass | 354.40 g·mol−1 |
| Density | 1.24 g/cm3[1] |
| Melting point | 157–159 °C (315–318 °F; 430–432 K) [1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Xanthohumol is a natural product found in the female inflorescences of Humulus lupulus, also known as hops. This compound is also found in beer and belongs to a class of compounds that contribute to the bitterness and flavor of hops.[2] Xanthohumol is a prenylated chalconoid, biosynthesized by a type III polyketide synthase (PKS) and subsequent modifying enzymes.[3][4]
Biosynthesis
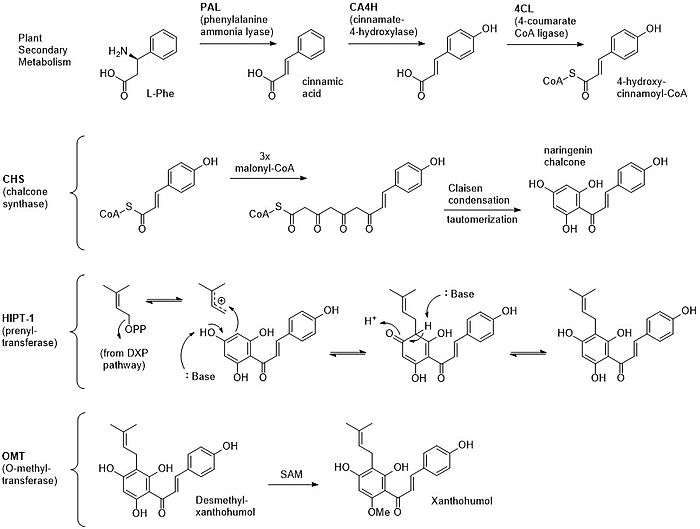
Xanthohumol is a prenylated chalconoid derived from a plant type III PKS, and is synthesized in the glandular trichromes of hop cones.[2] L-Phenylalanine serves as the starting material, which is converted to cinnamic acid by the PLP-dependent phenylalanine ammonia lyase.[5][6] Cinnamic acid is oxidized by cinnamate-4-hydroxylase and loaded onto Coenzyme A (CoA) by 4-coumarate CoA ligase to yield 4-hydroxy-cinnamoyl CoA, the starter unit for PKS extension.[5][6] This molecule is extended three times with malonyl CoA, cyclized through a Claisen condensation, and aromatized through tautomerization to form naringenin chalcone (chalconaringenin).[5] This intermediate has the potential to form a variety of different products depending on the enzymes that modify the core structure.[2][5] In the case of xanthohumol, a prenyltransferase called Humulus lupulus prenyltransferase 1 (HlPT-1) attaches a molecule of dimethylallyl pyrophosphate from the DXP pathway.[7] HlPT-1 has a broad substrate specificity and also participates in making other prenylated flavonoids in the hop plant.[7] Finally, an O-methyltransferase methylates a phenol substituent using S-adenosyl methionine.[6] Total syntheses of xanthohumol and derivatives have been achieved, though extraction from hops remains a primary source.[8][9]
Beer
In commercial beers, the concentration of xanthohumol ranges from about 2 μg/L – 1.2 mg/L.[10] During the brewing process, xanthohumol and other prenylated flavonoids are lost as they are converted to the corresponding flavanones.[11] Different hop varieties and different beers contain varying quantities of xanthohumol.[2]
Research
Xanthohumol is under basic research for its potential chemopreventive and anti-restenotic properties.[10][12][13]
See also
- Isoxanthohumol, the corresponding prenylated flavanone
- 8-Prenylnaringenin, a related prenylflavanoid with anti-estrogenic activity
- Alpha acids, a class of bitter compounds in hops
- Myrcene, humulene, and caryophyllene, essential oils in hops
References
- 1 2 Xanthohumol from hop (Humulus lupus), Santa Cruz Biotechnology
- 1 2 3 4 Stevens, Jan F.; Page, Jonathan E. (May 2004). "Xanthohumol and related prenylflavonoids from hops and beer: to your good health!". Phytochemistry. 65 (10): 1317–1330. doi:10.1016/j.phytochem.2004.04.025. ISSN 0031-9422. PMID 15231405.
- ↑ Goese, Markus; Kammhuber, Klaus; Bacher, Adelbert; Zenk, Meinhart H.; Eisenreich, Wolfgang (1999-07-15). "Biosynthesis of bitter acids in hops". European Journal of Biochemistry. 263 (2): 447–454. doi:10.1046/j.1432-1327.1999.00518.x. ISSN 1432-1033.
- ↑ Wang, Guodong; Tian, Li; Aziz, Naveed; Broun, Pierre; Dai, Xinbin; He, Ji; King, Andrew; Zhao, Patrick X.; Dixon, Richard A. (November 2008). "Terpene biosynthesis in glandular trichomes of hop". Plant Physiology. 148 (3): 1254–1266. doi:10.1104/pp.108.125187. ISSN 0032-0889. PMC 2577278. PMID 18775972.
- 1 2 3 4 M.,, Dewick, Paul. Medicinal natural products : a biosynthetic approach. ISBN 9780470741689. OCLC 259265604.
- 1 2 3 Nagel, Jana; Culley, Lana K.; Lu, Yuping; Liu, Enwu; Matthews, Paul D.; Stevens, Jan F.; Page, Jonathan E. (January 2008). "EST analysis of hop glandular trichomes identifies an O-methyltransferase that catalyzes the biosynthesis of xanthohumol". The Plant Cell. 20 (1): 186–200. doi:10.1105/tpc.107.055178. ISSN 1040-4651. PMC 2254931. PMID 18223037.
- 1 2 Tsurumaru, Yusuke; Sasaki, Kanako; Miyawaki, Tatsuya; Uto, Yoshihiro; Momma, Takayuki; Umemoto, Naoyuki; Momose, Masaki; Yazaki, Kazufumi (2012-01-06). "HlPT-1, a membrane-bound prenyltransferase responsible for the biosynthesis of bitter acids in hops". Biochemical and Biophysical Research Communications. 417 (1): 393–398. doi:10.1016/j.bbrc.2011.11.125. ISSN 1090-2104. PMID 22166201.
- ↑ Khupse, Rahul S.; Erhardt, Paul W. (2007-09-01). "Total Synthesis of Xanthohumol". Journal of Natural Products. 70 (9): 1507–1509. doi:10.1021/np070158y. ISSN 0163-3864.
- ↑ Zhang, Baoxin; Duan, Dongzhu; Ge, Chunpo; Yao, Juan; Liu, Yaping; Li, Xinming; Fang, Jianguo (2015-02-26). "Synthesis of Xanthohumol Analogues and Discovery of Potent Thioredoxin Reductase Inhibitor as Potential Anticancer Agent". Journal of Medicinal Chemistry. 58 (4): 1795–1805. doi:10.1021/jm5016507. ISSN 0022-2623.
- 1 2 Gerhäuser, Clarissa (September 2005). "Beer constituents as potential cancer chemopreventive agents". European Journal of Cancer. 41 (13): 1941–1954. doi:10.1016/j.ejca.2005.04.012. ISSN 0959-8049. PMID 15953717.
- ↑ Stevens, Jan F.; Taylor, Alan W.; Clawson, Jeff E.; Deinzer, Max L. (1999-06-01). "Fate of Xanthohumol and Related Prenylflavonoids from Hops to Beer". Journal of Agricultural and Food Chemistry. 47 (6): 2421–2428. doi:10.1021/jf990101k. ISSN 0021-8561.
- ↑ Gerhauser, Clarissa; Alt, Axel; Heiss, Elke; Gamal-Eldeen, Amira; Klimo, Karin; Knauft, Jutta; Neumann, Isabell; Scherf, Hans-Rudolf; Frank, Norbert (September 2002). "Cancer chemopreventive activity of Xanthohumol, a natural product derived from hop". Molecular Cancer Therapeutics. 1 (11): 959–969. ISSN 1535-7163. PMID 12481418.
- ↑ Liu R, Heiss EH, Schachner D, Jiang B, Liu W, Breuss JM, Dirsch VM, Atanasov AG. Xanthohumol Blocks Proliferation and Migration of Vascular Smooth Muscle Cells in Vitro and Reduces Neointima Formation in Vivo. J Nat Prod. 2017 Jun 19. doi: 10.1021/acs.jnatprod.7b00268.