Wurtz–Fittig reaction
| Wurtz–Fittig reaction | |
|---|---|
| Named after | Charles-Adolphe Wurtz Wilhelm Rudolph Fittig |
| Reaction type | Coupling reaction |
| Identifiers | |
| Organic Chemistry Portal | wurtz-fittig-reaction |
The Wurtz–Fittig reaction is the chemical reaction of aryl halides with alkyl halides and sodium metal in the presence of dry ether to give substituted aromatic compounds.[1] Charles-Adolphe Wurtz reported what is now known as the Wurtz reaction in 1855,[2][3] involving the formation of a new carbon-carbon bond by coupling two alkyl halides.[4][5] Work by Rudolph Fittig in the 1860s extended the approach to the coupling of an alkyl halide with an aryl halide.[6][7] This modification of the Wurtz reaction is considered a separate process and is named for both scientists.[1]
The reaction works best for forming asymmetrical products if the halide reactants are somehow separate in their relative chemical reactivities. One way to accomplish this is to form the reactants with halogens of different periods. Typically the alkyl halide is made more reactive than the aryl halide, increasing the probability that the alkyl halide will form the organosodium bond first and thus act more effectively as a nucleophile toward the aryl halide.[8] Typically the reaction is used for the alkylation of aryl halides; however, with the use of ultrasound the reaction can also be made useful for the production of biphenyl compounds.[9]
Mechanism
There are two approaches to describing the mechanism of the Wurtz-Fittig reaction.[10][11] The first involves the sodium-mediated formation of both alkyl and aryl radicals. The alkyl and aryl radicals then combine to form a substituted aromatic compound.
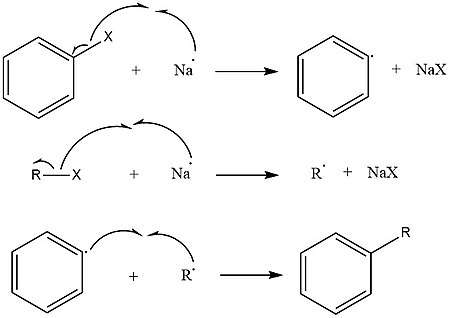
The second approach involves the formation of an intermediate organo-alkali compound followed by nucleophilic attack of the alkyl halide.

There is empirical evidence for both approaches. The free radical mechanism is supported by the observation of side products whose formation cannot be explained by an organo-alkali mechanism.[12] In a reaction between sodium and cholorobenzene, Bachmann and Clarke[12] find that one of the many side products is triphenylene. They contend that the only way to explain the formation of triphenylene is through a free radical mechanism.
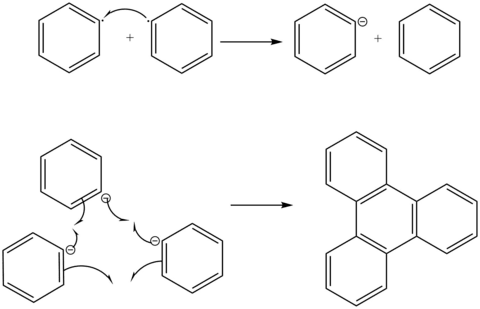
In this mechanism, two free phenyl radicals react to form benzene and a free phenylene anion. The three phenylene anions then combine via a radical mechanism to form the triphenylene molecule. Bachmann and Clarke[12] consider the occurrence of triphenylene as evidence that the Wurtz-Fittig reaction happens through a radical mechanism.
The organo-alkali mechanism is supported by indirect evidence which shows that an organo-alkali intermediate actually forms during the reaction.[11] This has been observed my many investigators.[10] For example, Shoruguin[13] shows that bubbling carbon dioxide through a mixture of sodium and isobutyl bromide results in the formation of 3-methylbutanoic acid.

The formation of 3-methylbutanoic acid follows from a nucelophilic attack of carbon dioxide by an organosodium compound. These results suggest that Wurtz-Fittig reaction occurs via the formation of an organoalkali compound since the reaction conditions are similar.
Use of Other Metals
The Wurtz-Fittig Reaction can be conducted using metals other than sodium. Some examples include potassium, iron, copper, and lithium.[14] When lithium is used, the reaction occurs with appreciable yield only under ultrasound.[15] Ultrasound is known to cleave halogen atoms from aryl and alkyl halides via a free radical mechanism[16]
Applications
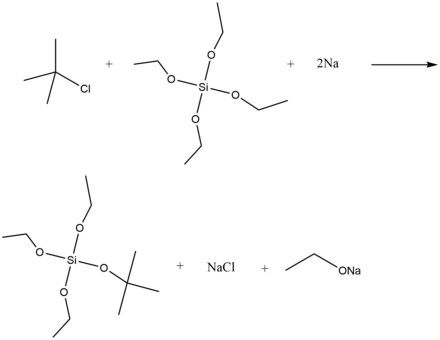
The Wurtz-Fittig Reaction has limited applicability since side reactions such as rearrangements and eliminations are prevalent.[14] However, the reaction is useful for the laboratory synthesis of organosilicon compounds, although there are challenges in adapting the procedure to a large-scale industrial process.[17] Organosilicon compounds successfully synthesized via the Wurtz-Fittig reaction include silylated calixarenes,[18] t-Butylsilicon compounds,[19] and vinylsilanes.[20] For example, t-butyltriethoxysilane can be prepared via the Wurtz-Fitting reaction by combining tetraoxysilane, t-butyl choride and molten sodium. The reaction proceeds with a 40% yield.[19]
See also
References
- 1 2 Zerong Wang (2010). "Wurtz-Fittig Reaction". Comprehensive Organic Name Reactions and Reagents. 686. pp. 3100–3104. doi:10.1002/9780470638859.conrr686. ISBN 9780470638859.
- ↑ Wurtz, Adolphe (1855). "Sur une Nouvelle Classe de Radicaux Organiques" [On a New Class of Organic Radicals]. Annales de Chimie et de Physique (in French). 44: 275-312.
- ↑ Wurtz, A. (1855). "Ueber eine neue Klasse organischer Radicale" [About a new class of organic radicals]. Justus Liebigs Annalen der Chemie (in German). 96 (3): 364–375. doi:10.1002/jlac.18550960310.
- ↑ Zerong Wang (2010). "Wurtz Synthesis (Wurtz Reaction, Wurtz Reductive Coupling)". Comprehensive Organic Name Reactions and Reagents. 685. pp. 3094–3099. doi:10.1002/9780470638859.conrr685. ISBN 9780470638859.
- ↑ Kantchev, Eric Asssen B.; Organ, Michael G. (2014). "48.1.2.4 Method 4: Reductive Coupling of Alkyl Halides". In Hiemstra, H. Alkanes. Science of Synthesis: Houben-Weyl Methods of Molecular Transformations. 48. Georg Thieme Verlag. ISBN 9783131784810.
- ↑ Tollens, Bernhard; Fittig, Rudolph (1864). "Ueber die Synthese der Kohlenwasserstoffe der Benzolreihe" [On the synthesis of the hydrocarbons of the benzene series]. Justus Liebigs Annalen der Chemie (in German). 131 (3): 303–323. doi:10.1002/jlac.18641310307.
- ↑ Fittig, Rudolph; König, Joseph (1867). "Ueber das Aethyl- und Diäthylbenzol" [About ethyl- and diethylbenzene]. Justus Liebigs Annalen der Chemie (in German). 144 (3): 277–294. doi:10.1002/jlac.18671440308.
- ↑ Desai, K.R. (2008). Organic Name Reactions. Jaipur I India: Oxford Book Company. p. 259. ISBN 9788189473327.
- ↑ Laue, Thomas & Plagens, Andreas (2005). Named Organic Reactions 2nd Ed. Wolfsburg, Germany: John Wiely & Sons, Ltd. p. 305. ISBN 9780470010402.
- 1 2 Wooster, Charles Bushnell (August 1932). "Organo-alkali Compounds". Chemical Reviews. 11 (1): 1–91. doi:10.1021/cr60038a001. ISSN 0009-2665.
- 1 2 Gilman, Henry; Wright, George F. (July 1933). "The Mechanism of the Wurtz—Fittig Reaction. The Direct Preparation of an Organosodium (Potassium) Compound from an RX Compound". Journal of the American Chemical Society. 55 (7): 2893–2896. doi:10.1021/ja01334a044. ISSN 0002-7863.
- 1 2 3 Bachmann, W. E.; Clarke, H. T. (August 1927). "The Mechanism of the Wurtz-Fitting Reaction". Journal of the American Chemical Society. 49 (8): 2089–2098. doi:10.1021/ja01407a038. ISSN 0002-7863.
- ↑ Schoruigin: Ber. 41, 2711-7 (1908); ibid. 43, 1938-42 (1910).
- 1 2 Smith, Michael; March, Jerry (2007). March's advanced organic chemistry : reactions, mechanisms, and structure (6th ed.). Hoboken, N.J.: Wiley-Interscience. ISBN 0471720917. OCLC 69020965.
- ↑ Byung Hee Han; Philip Boudjouk (1981). "Organic sonochemistry. Ultrasound-promoted coupling of organic halides in the presence of lithium wire". Tetrahedron Letters. 22 (29): 2757–2758. doi:10.1016/S0040-4039(01)90544-1. ISSN 0040-4039.
- ↑ S. Prakash; J.D. Pandey (1965). "Sonocleavage of halogens from aliphatic chains and aromatic rings". Tetrahedron. 21 (4): 903–908. doi:10.1016/0040-4020(65)80026-6. ISSN 0040-4020.
- ↑ Bassett, E. A.; Emblem, H. G.; Frankel, M.; Ridge, D. (May 1948). "The use of the wurtz-fittig reaction in the preparation of organo-substituted silanes". Journal of the Society of Chemical Industry. 67 (5): 177–179. doi:10.1002/jctb.5000670503. ISSN 0368-4075.
- ↑ Hudrlik, Paul F.; Arasho, Wondwossen D.; Hudrlik, Anne M. (October 2007). "The Wurtz−Fittig Reaction in the Preparation of C-Silylated Calixarenes". The Journal of Organic Chemistry. 72 (21): 8107–8110. doi:10.1021/jo070660n. ISSN 0022-3263.
- 1 2 CHAPPELOW, C. C.; ELLIOTT, R. L.; GOODWIN, J. T. (April 1962). "Synthesis of t-Butylsilicon Compounds by the Wurtz-Fitting Reaction1". The Journal of Organic Chemistry. 27 (4): 1409–1414. doi:10.1021/jo01051a069. ISSN 0022-3263.
- ↑ Adam, Waldemar; Richter, Markus J. (1994). "One-Pot Synthesis of α-Trimethylsilyl Enones from Vinylsilanes". Synthesis. 1994 (02): 176–180. doi:10.1055/s-1994-25433. ISSN 0039-7881.
