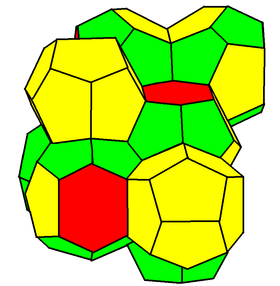
Weaire–Phelan structure
| Weaire–Phelan structure | |
|---|---|
 | |
| Space group Fibrifold notation Coxeter notation | Pm3n (223) 2o [[4,3,4]+] |
In geometry, the Weaire–Phelan structure is a complex 3-dimensional structure representing an idealised foam of equal-sized bubbles. In 1993, Trinity College Dublin physicist Denis Weaire and his student Robert Phelan found in computer simulations of foam that this structure was a better solution of the "Kelvin problem" than the previous best-known solution, the Kelvin structure.[1]
The Kelvin conjecture
In 1887, Lord Kelvin asked how space could be partitioned into cells of equal volume with the least area of surface between them, i.e., what was the most efficient bubble foam?[2] This problem has since been referred to as the Kelvin problem.
He proposed a foam, based on the bitruncated cubic honeycomb, which is called the Kelvin structure. This is the convex uniform honeycomb formed by the truncated octahedron, which is a 14-faced space-filling polyhedron (a tetradecahedron), with 6 square faces and 8 hexagonal faces. To conform to Plateau's laws governing the structures of foams, the hexagonal faces of Kelvin's variant are slightly curved.
The Kelvin conjecture is that this structure solves the Kelvin problem: that the foam of the bitruncated cubic honeycomb is the most efficient foam. The Kelvin conjecture was widely believed, and no counter-example was known for more than 100 years, until it was disproved by the discovery of the Weaire–Phelan structure.
In 2009, Ruggero Gabbrielli[3] published a way to use the Swift–Hohenberg equation to find candidate solutions to the Kelvin Problem on minimal surfaces.[4][5]
Description of Weaire–Phelan structure
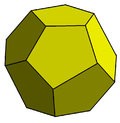
The Weaire–Phelan structure differs from Kelvin's in that it uses two kinds of cells, although they have equal volume.
From a topological and symmetrical point of view, one is a pyritohedron, an irregular dodecahedron with pentagonal faces, possessing tetrahedral symmetry (Th).
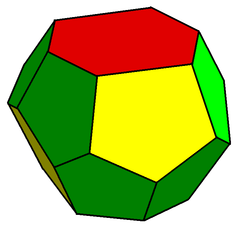
The second is a form of truncated hexagonal trapezohedron, a species of tetrakaidecahedron with two hexagonal and twelve pentagonal faces, in this case only possessing two mirror planes and a rotoreflection symmetry.
Like the hexagons in the Kelvin structure, the pentagons in both types of cells are slightly curved. The surface area of the Weaire–Phelan structure is 0.3% less than that of the Kelvin structure. It has not been proved that the Weaire–Phelan structure is optimal. Experiments have also shown that, with favorable boundary conditions, equal-volume bubbles spontaneously self-assemble into the A15 phase, whose atoms coincide with the centroids of the polyhedra in the Weaire–Phelan structure.[6][7]

Polyhedral approximation
The polyhedral honeycomb associated with the Weaire–Phelan structure (obtained by flattening the faces and straightening the edges) is also referred to loosely as the Weaire–Phelan structure. It was known well before the Weaire–Phelan structure was discovered, but the application to the Kelvin problem was overlooked.[8]
It is found in two related geometries of crystal structure in chemistry.
Where the components of the crystal lie at the centres of the polyhedra it forms one of the Frank–Kasper phases.[9]
Where the components of the crystal lie at the corners of the polyhedra, it is known as the "Type I clathrate structure". Gas hydrates formed by methane, propane and carbon dioxide at low temperatures have a structure in which water molecules lie at the nodes of the Weaire–Phelan structure and are hydrogen bonded together, and the larger gas molecules are trapped in the polyhedral cages. Some alkali metal silicides and germanides also form this structure (Si/Ge at nodes, alkali metals in cages), as does the silica mineral melanophlogite (silicon at nodes, linked by oxygen along edges). Melanophlogite is a metastable form of SiO2 that is stabilized in this structure because of gas molecules trapped in the cages. The International Zeolite Association uses the symbol MEP to indicate the framework topology of melanophlogite.
Applications
The Weaire–Phelan structure is the inspiration for the design of the Beijing National Aquatics Centre for the 2008 Olympics in Beijing in China.[10] The resulting structural support system is inherently strong and lightweight. As all joints in the structure are close to tetrahedral angles, the framework fills a large volume of space with a reduced amount of material, similar to a hexagonal honeycomb in two dimensions.
See also
- Honeycomb conjecture, a two-dimensional version of the Kelvin conjecture.
- Minimal surface
- Soap bubble
References
- ↑ Weaire, D.; Phelan, R. (1994), "A counter-example to Kelvin's conjecture on minimal surfaces", Phil. Mag. Lett., 69: 107–110, doi:10.1080/09500839408241577 .
- ↑ Lord Kelvin (Sir William Thomson) (1887), "On the Division of Space with Minimum Partitional Area" (PDF), Philosophical Magazine, 24 (151): 503, doi:10.1080/14786448708628135 .
- ↑ Gabbrielli, Ruggero. "Ruggero Gabbrielli - Google Scholar Citations". scholar.google.com.
- ↑ Gabbrielli, Ruggero (1 August 2009). "A new counter-example to Kelvin's conjecture on minimal surfaces". Philosophical Magazine Letters. 89 (8): 483–491. doi:10.1080/09500830903022651. ISSN 0950-0839. Retrieved 4 July 2017.
- ↑ Freiberger, Marianne (24 September 2009). "Kelvin's bubble burst again | plus.maths.org". Plus Magazine. University of Cambridge. Retrieved 4 July 2017.
- ↑ Gabbrielli, R.; Meagher, A.J.; Weaire, D.; Brakke, K.A.; Hutzler, S. (2012), "An experimental realization of the Weaire-Phelan structure in monodisperse liquid foam", Phil. Mag. Lett., 92: 1–6, doi:10.1080/09500839.2011.645898 .
- ↑ Ball, Philip (2011), "Scientists make the 'perfect' foam: Theoretical low-energy foam made for real", Nature, doi:10.1038/nature.2011.9504 .
- ↑ A diagram can be found in Pauling, Linus (1960). The Nature of the Chemical Bond (3rd ed.). Cornell University Press. p. 471. , as shown on Ken Brakke's page.
- ↑ Frank, F. C.; Kasper, J. S. (1958), "Complex alloy structures regarded as sphere packings. I. Definitions and basic principles", Acta Crystallogr., 11, doi:10.1107/s0365110x58000487 . Frank, F. C.; Kasper, J. S. (1959), "Complex alloy structures regarded as sphere packings. II. Analysis and classification of representative structures", Acta Crystallogr., 12, doi:10.1107/s0365110x59001499 .
- ↑ Fountain, Henry (August 5, 2008), "A Problem of Bubbles Frames an Olympic Design", New York Times .
External links
- 3D models of the Weaire–Phelan, Kelvin and P42a structures
- Weaire–Phelan structure unfolded dodecahedron and tetrakaidecahedron in .pdf / .dxf formats
- An image of the Weaire–Phelan structure
- Weaire–Phelan Bubbles page with illustrations and freely downloadable 'nets' for printing and making models.
- Beating Kelvin's Partition of Space
- "Weaire-Phelan Smart Modular Space Settlement", Alexandru Pintea, 2017, Individual First Prize NASA Ames Space Settlement Contest:
