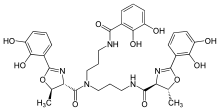
Vibriobactin
 | |
| Names | |
|---|---|
| IUPAC name
N-[3-(2,3-Dihydroxybenzamido)propyl]-1,3-bis[2-(2,3-dihydroxyphenyl)-trans-5-methyl-2-oxazoline-4-carboxamido]propane | |
| Identifiers | |
| Properties | |
| C35H39N5O11 | |
| Molar mass | 705.72 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Vibriobactin is a catechol siderophore that helps the microbial system to acquire iron. It was first isolated from Vibrio cholerae.[1]
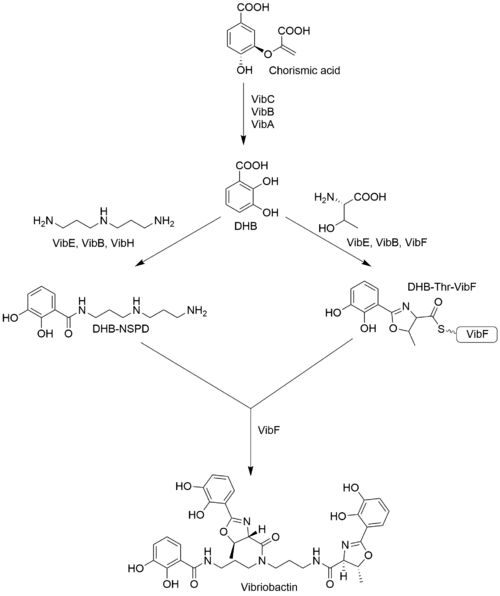
Structure and biosynthesis
The components of vibriobactin are three 2,3-dihydroxybenzoic acid (DHB), two threonine (Thr), and one norspermidine (NSPD). DHB is synthesized from chorismic acid by a series of enzymes: VibA, VibB, and VibC. DHB is linked to NSPD by VibE, VibB and VibH in order and forms DHB-NSPD.[2] On the other hand, DHB performs condensation and cyclization with Thr by VibE, VibB, and VibF to form the heterocyclic molecule linked on VibF: DHB-Thr-VibF. DHB-NSPD and DHB-Thr-VibF are then put together by VibF to form vibriobactin.[3]
References
- ↑ Griffiths, Gary L.; Sigel, Suzanne P.; Payne, Shelley M.; Neilands, J. B. (1984). "Vibriobactin, a Siderophore from Vibrio cholerae". The Journal of Biological Chemistry. 259 (1): 383.
- ↑ Keating, Thomas A.; Marshall, C. Gary; Walsh, Christopher T. (2000). "Vibriobactin Biosynthesis in Vibrio cholerae: VibH Is an Amide Synthase Homologous to Nonribosomal Peptide Synthetase Condensation Domains". Biochemistry. 39: 15514.
- ↑ Marshall, C. Gary; Burkart, Michael D.; Keating, Thomas A.; Walsh, Christopher T. (2001). "Heterocycle Formation in Vibriobactin Biosynthesis: Alternative Substrate Utilization and Identification of a Condensed Intermediate". Biochemistry. 40: 10656.
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.