Veliparib
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| ECHA InfoCard |
100.206.770 |
| Chemical and physical data | |
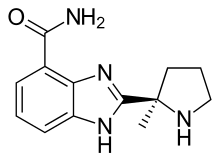
| Formula | C13H16N4O |
| Molar mass | 244.29 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
Veliparib (ABT-888)[1] is a potential anti-cancer drug acting as a PARP inhibitor. It kills cancer cells by blocking a protein called PARP, thereby preventing the repair of DNA or genetic damage in cancer cells and possibly making them more susceptible to anticancer treatments. Veliparib may make whole brain radiation treatment work more effectively against brain metastases from NSCLC. It has been shown to potentiate the effects of many chemotherapeutics, and as such has been part of many combination clinical trials.[2]
Development
Veliparib is being developed by AbbVie. It was derived from a prior lead compound (A 620223). The FDA awarded orphan drug status in November 2016 for NSCLC.[2]
Clinical trials
As of 2017, 96 clinical trials involving veliparib had been registered with the FDA.[5] It was included in the I-SPY2 breast cancer trial.[6]
Numerous phase I clinical trials are in progress.[7] Over 40 phase II clinical trials have been registered, for indications such as metastatic melanoma,[8] breast cancer,[9] NSCLC, prostate cancer[10] and brain tumors associated with metastatic primary tumors.
Combination trials have evaluated velibarib in combination with doxorubicin, temozolomide, topotecan, carboplatin, paclitaxel, pemetrexed, cyclophosphamide, gemcitabine, and others.[11]
By June 2014 it was in three phase III trials, for advanced ovarian cancer, triple-negative breast cancer and in non-small cell lung cancer (NSCLC).[12] In 2017, AbbVie reported that veliparib failed to improve outcomes in the triple-negative breast cancer and NSCLC trials.[13]
References
- ↑ "ABT-888, an Orally Active Poly(ADP-Ribose) Polymerase Inhibitor that Potentiates DNA-Damaging Agents in Preclinical Tumor Models" May 2007
- 1 2 Veliparib drug profile
- ↑ http://www.cancer.gov/drugdictionary/?CdrID=496464
- ↑ "ABT-888, an Orally Active Poly(ADP-Ribose) Polymerase Inhibitor that Potentiates DNA-Damaging Agents in Preclinical Tumor Models", 2007
- ↑ https://clinicaltrials.gov/ct2/results?term=veliparib
- ↑ "Breast cancer study aims to speed drugs, cooperation", March 2010
- ↑ http://clinicaltrialsfeeds.org/clinical-trials/results/term=Drug:+ABT-888
- ↑ "A Study Evaluating Efficacy of ABT-888 in Combination With Temozolomide in Metastatic Melanoma"
- ↑ "ABT-888 and Temozolomide for Metastatic Breast Cancer"
- ↑ Abiraterone Acetate and Prednisone With or Without Veliparib in Treating Patients With Metastatic Castration-Resistant Prostate Cancer
- ↑ https://clinicaltrials.gov/ct2/results?term=veliparib
- ↑ AbbVie takes PARP inhibitor into third phase III trial. June 2014
- ↑ "AbbVie PARP inhibitor veliparib flunks two phase 3 trials". Fierce Biotech. April 20, 2017.