Uranium(III) iodide
 | |
| Identifiers | |
|---|---|
| ECHA InfoCard | 100.033.992 |
| Properties | |
| UI3 | |
| Molar mass | 618.74232 g/mol |
| Appearance | black solid |
| Density | 6.78 g/cm3[1] |
| Melting point | 766[2] °C (1,411 °F; 1,039 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Uranium triiodide is an inorganic compound with the chemical formula UI3. It is a black solid.
Production
Uranium triiodide can be obtained from the direct reaction of its constituent elements:[3]
- 2 U + 3 I2 → 2 UI3
When the reaction is conducted in tetrahydrofuran (thf), the product is the blue UI3(thf)4.[4]
Properties
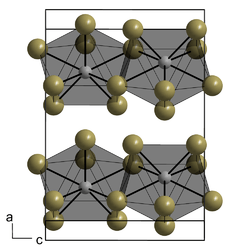
It crystallizes in the orthorhombic crystal system (plutonium tribromide-type) in the space group Ccmm with the lattice parameters a = 432.8 pm, b = 1401.1 pm, and c = 1000.5 pm and four formula units per unit cell.[1]
Uranium triiodide is Lewis acidic.[5]
References
- 1 2 J. H. Levy, J. C. Taylor, P. W. Wilson: "The Structure of Uranium(III) Triiodide by Neutron Diffraction", in: Acta Crystallogr. B, 1975, 31, S. 880–882 (doi:10.1107/S0567740875003986).
- ↑ Arnold F. Holleman, Nils Wiberg: Lehrbuch der Anorganischen Chemie, 102. Auflage, de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1, S. 1969.
- ↑ Georg Brauer (ed.): Handbuch der Präparativen Anorganischen Chemie. 3rd edition. Vol. 2, Enke, Stuttgart 1978, ISBN 3-432-87813-3, p. 1218.
- ↑ David L. Clark, Alfred P. Sattelberger (1997). "Lewis Base Adducts of Uranium Triiodide and Tris[Bis(Trimethylsilyl)Amido]Uranium". Inorg. Synth. 31: 307–315. doi:10.1002/9780470132623.ch55.
- ↑ Jacqueline Collin, Leonor Maria, Isabel Santos: "Uranium Iodides as Catalysts for Diels–Alder Reactions", in: Journal of Molecular Catalysis A: Chemical, 2000, 160 (2), pp. 263–267 (doi:10.1016/S1381-1169(00)00257-0).
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.