Tsuji–Wilkinson decarbonylation reaction
| Tsuji–Wilkinson decarbonylation | |
|---|---|
| Named after | Jiro Tsuji Geoffrey Wilkinson |
| Reaction type | Decarbonylation |
The Tsuji–Wilkinson decarbonylation reaction is a method for the decarbonylation of aliphatic, aromatic, as well as α,β-unsaturated aldehydes into the corresponding C(sp3)–H or C(sp2)–H bonds.[1][2][3][4] Further, the Tsuji–Wilkinson decarbonylation may be applied to acyl halides.[2]
- RC(O)X + RhCl(PPh3)3 → RCX + RhCl(CO)(PPh3)2 + PPh3
Although decarbonylation is effected by several transition metal complexes (e.g. palladium complexes),[5] Wilkinson's catalyst is the most efficient. Unfortunately, the reaction usually requires stoichiometric amounts of Wilkinson’s catalyst due the inertness of the product bis(triphenylphosphine)rhodium carbonyl chloride. If the reaction is carried out above 200 °C, carbon monoxide may be liberated efficiently enough and the reaction may proceed with catalytic amount of complex. Other transition metals such as Iridium(I) may obviate this issue.[6]
The Tsuji–Wilkinson decarbonylation has been applied to specialized syntheses. The reaction proceeds under mild conditions and is highly stereospecific. In addition to aliphatic, aromatic, and α,β-unsaturated aldehydes, acyl nitriles and 1,2-diketones are also suitable substrates
History
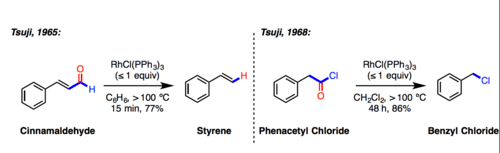
In 1965, Jiro Tsuji reported that stoichiometric amounts of Wilkinson's Catalytst (RhCl(PPh3)3) was capable of decarbonylating cinnamaldehyde.[1] In 1968, Tsuji again showed Wilkinson's catalyst can decarbonylate phenacyl chloride to benzyl chloride.[2]
Other developments in reaction improvement, see below: Efforts to render a low temperature catalytic Tsuji–Wilkinson decarbonylation.
Catalytic cycle
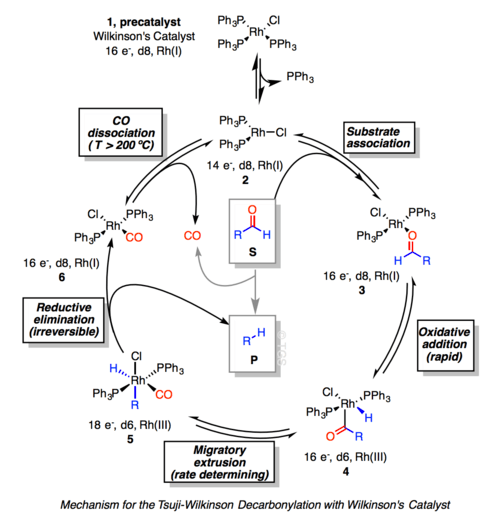
With aldehyde substrates, the catalytic cycle is assumed to begin with dissociation of triphenylphosphine to give the 14 electron RhCl(PPh3)2. Oxidative addition of the aldehyde gives a d6 acyl Rh(III)-hydride intermediate. Migratory extrusion of CO proceed to form the 18-electron d6 Rh(III) species, 5. In the catalytic variant of the Tsuji–Wilkinson decarbonylation, RhCl(CO)(PPh3)2 evolves CO above 200 °C, thereby regenerating RhCl(PPh3)n. Otherwise, the reaction mechanism halts by formation of the thermodynamically stable 6 (the stoichiometric variant).[6]
Synthetic applications
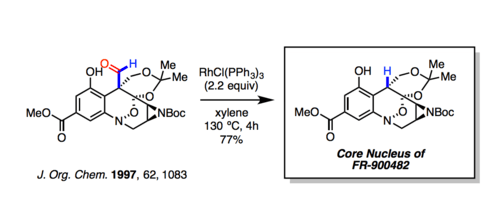
The Tsuji–Wilkinson decarbonylation has been used to prepared numerous natural products. One example is the synthesis of the core nucleus of FR-900482.[7] Note that the ester is unaffected by the rhodium reagent.
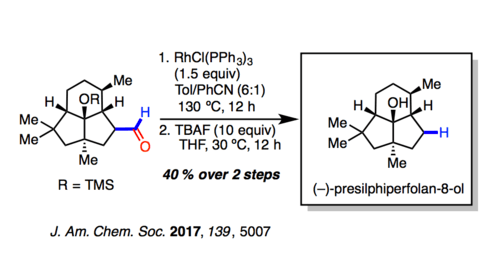
The Tsuji–Wilkinson decarbonylation is employed in the penultimate step of the synthesis of (–)-presilphiperfolan-8-ol.[8] They comment “Of note in these final steps, separate reduction and oxidation steps proceeded in inferior yield in generating 38 (70% versus 93%),32 while the Rh(PPh3)3Cl operation proceeded smoothly when conducted on small scale (∼15 mg). In total, the synthesis required 13 steps from commercial [starting material], and ∼15 mg of [(–)-presilphiperfolan-8-ol] has been prepared with spectral properties and optical rotations matching that of the natural isolate.”
Efforts to render a low temperature catalytic Tsuji–Wilkinson decarbonylation
The Tsuji–Wilkinson reaction suffers from the requirement for stoichiometric Wilkinson's catalyst or the need for high temperatures to regenerate the rhodium reagent. The ideal Tsuji–Wilkinon decarbonylation would be typified by catalytic (e.g. 10 mol% catalyst) at mild temperatures. The reaction has been carried out in flow conditions at low temperatures in which a biphasic liquid-gas flow decarbonylation was developed employing N2 as a gas carrier.[9] However, the temperature required for this reaction is 200 °C.
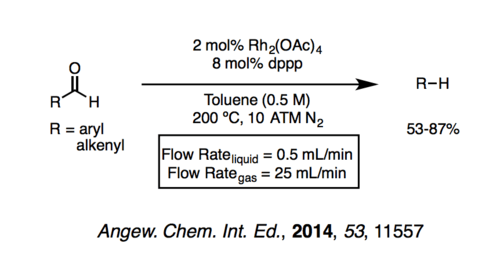
Significant improvements of the Tsuji–Wilkinson decarbonylation have been made by using cationic rhodium complexes with chelating bisphosphines (which increase the rate of reductive elimination and lower the energetic barrier for CO extrusion).[10] These complexes have less electron density, therefore reducing backbonding into CO and therefore have significantly weaker metal–CO bonds. Improvements to this area of research are actively underway.
References
- 1 2 Tsuji, Jiro; Ohno, Kiyotaka (January 1965). "Organic syntheses by means of noble metal compounds XXI. Decarbonylation of aldehydes using rhodium complex". Tetrahedron Letters. 6 (44): 3969–3971. doi:10.1016/S0040-4039(01)89127-9.
- 1 2 3 Ohno, Kiyotaka; Tsuji, Jiro (1968). "Organic synthesis by means of noble metal compounds. XXXV. Novel decarbonylation reactions of aldehydes and acyl halides using rhodium complexes". Journal of the American Chemical Society. 90 (1): 99–107. doi:10.1021/ja01003a018.
- ↑ Tsuji, Jiro; Ohno, Kiyotaka; Kajimoto, Tsunesuke (January 1965). "Organic syntheses by means of noble metal compounds XX. Decarbonylation of acyl chloride and aldehyde catalyzed by palladium and its relationship with the rosenmund reduction". Tetrahedron Letters. 6 (50): 4565–4568. doi:10.1016/S0040-4039(01)89065-1.
- ↑ Synthesis, 1969, 157
- ↑ Laszlo Kurti; Barbara Czako (2005). Strategic Applications of Named Reactions in Organic Synthesis: Background and Detailed Mechanism. ISBN 0124297854.
- 1 2 Doughty, D. H.; Pignolet, L. H. (October 1978). "Catalytic decarbonylation of aldehydes". Journal of the American Chemical Society. 100 (22): 7083–7085.
- ↑ Ziegler, Frederick E.; Belema, Makonen (February 1997). "Chiral Aziridinyl Radicals: An Application to the Synthesis of the Core Nucleus of FR-900482". The Journal of Organic Chemistry. 62 (4): 1083–1094. doi:10.1021/jo961992n.
- ↑ Hu, Pengfei; Snyder, Scott A. (29 March 2017). "Enantiospecific Total Synthesis of the Highly Strained (−)-Presilphiperfolan-8-ol via a Pd-Catalyzed Tandem Cyclization". Journal of the American Chemical Society. 139 (14): 5007–5010. doi:10.1021/jacs.7b01454.
- ↑ Gutmann, Bernhard; Elsner, Petteri; Glasnov, Toma; Roberge, Dominique M.; Kappe, C. Oliver (20 October 2014). "Shifting Chemical Equilibria in Flow-Efficient Decarbonylation Driven by Annular Flow Regimes". Angewandte Chemie International Edition. 53 (43): 11557–11561. doi:10.1002/anie.201407219.
- ↑ Kreis, Michael; Palmelund, Anders; Bunch, Lennart; Madsen, Robert (October 2006). "A General and Convenient Method for the Rhodium-Catalyzed Decarbonylation of Aldehydes". Advanced Synthesis & Catalysis. 348 (15): 2148–2154. doi:10.1002/adsc.200600228.