Trimethyl phosphite
 | |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Trimethyl phosphite | |||
| Other names
Trimethoxyphosphine | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChemSpider | |||
| ECHA InfoCard | 100.004.065 | ||
PubChem CID |
|||
| |||
| |||
| Properties | |||
| C3H9O3P | |||
| Molar mass | 124.08 | ||
| Appearance | colorless liquid | ||
| Odor | distinctive, pungent[1] | ||
| Density | 1.052 | ||
| Melting point | −78 °C (−108 °F; 195 K) | ||
| Boiling point | 111 °C (232 °F; 384 K) | ||
| reacts[1] | |||
| Vapor pressure | 24 mmHg (25°C)[1] | ||
| Hazards | |||
| Flash point | 28 °C; 82 °F; 301 K [1] | ||
| US health exposure limits (NIOSH): | |||
PEL (Permissible) |
none[1] | ||
REL (Recommended) |
TWA 2 ppm (10 mg/m3)[1] | ||
IDLH (Immediate danger) |
N.D.[1] | ||
| Related compounds | |||
Related compounds |
Dimethyl methylphosphonate | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
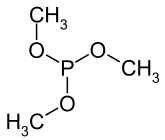
Trimethylphosphite is an organophosphorus compound with the formula P(OCH3)3, often abbreviated P(OMe)3. This colorless liquid is used as a ligand in organometallic chemistry and as a reagent in organic synthesis. The material also has a highly pungent smell. The molecule features a pyramidal phosphorus(III) center bound to three methoxy groups.
Synthesis and reactions
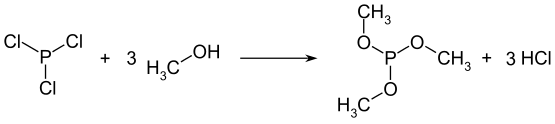
Trimethylphosphite is prepared from phosphorus trichloride:
It is susceptible to oxidation to trimethyl phosphate.
It reacts with a catalytic amount of methyl iodide in the Arbuzov reaction to give dimethyl methylphosphonate:
- P(OCH3) → CH3)P(O)(OCH2)
As a ligand, trimethylphosphite has a smaller cone angle and better acceptor properties relative to trimethylphosphine. A representative derivative is the colorless, tetrahedral complex Ni(P(OMe)3)4 (m.p. 108 °C).[2] The tridentate ligand called the Klaui ligand is derived from trimethylphosphite. The formation of this ligand illustrates the susceptibility of trimethylphosphite (and metal complexes thereof) to the Arbuzov reaction.
Trimethylphosphite is also used as a mild desulfurization reagent in organic synthesis, for example in the preparation of derivatives of tetrathiafulvalene.[3]
Toxicity
References
- 1 2 3 4 5 6 7 "NIOSH Pocket Guide to Chemical Hazards #0640". National Institute for Occupational Safety and Health (NIOSH).
- ↑ Ittel, Steven D.; Ittel, S. D.; Cushing, M. A. (1990). "Complexes of Nickel(0)". Inorganic Syntheses. Inorganic Syntheses. 28: 98–104. doi:10.1002/9780470132593.ch25. ISBN 978-0-471-52619-3.
- ↑ Jan Larsen and Christine Lenoir (1998). "2,2'-Bi-5,6-Dihydro-1,3-Dithiolo[4,5-b][1,4]dithiinylidene (BEDT-TTF)". Organic Syntheses. ; Collective Volume, 9, p. 72 .
- ↑ Svara, J.; Weferling, N.; Hofmann, T., "Phosphorus Compounds, Organic", Ullmann's Encyclopedia of Industrial Chemistry, Weinheim: Wiley-VCH, doi:10.1002/14356007.a19_545.pub2