Triflidic acid
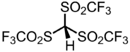 | |
| Names | |
|---|---|
| IUPAC name
Tris[(trifluoromethyl)sulfonyl]methane | |
| Other names
Triflidic acid, tris(triflyl)methane | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| |
| |
| Properties | |
| C4F9S3O6H | |
| Molar mass | 412.23 g/mol |
| Appearance | Colorless solid |
| Melting point | 69.2 °C (156.6 °F; 342.3 K) |
| Miscible | |
| Acidity (pKa) | –18.6 (aqueous, est.) [1] |
| Hazards | |
| Main hazards | Corrosive, eye irritant |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Triflidic acid (IUPAC name: tris[(trifluoromethyl)sulfonyl]methane, abbreviated formula: Tf3CH) is an organic superacid. It is one of the strongest known carbon acids and is among the strongest Brønsted acids in general, with an acidity exceeded only by the carborane acids. Notably, triflidic acid is estimated to have an acidity 104 times that of triflic acid (pKaaq ~ –14), as measured by its acid dissociation constant. It was first prepared in 1987 by Seppelt and Lutz by the following route:[2]
(1) Tf2CH2 + 2CH3MgBr → Tf2C(MgBr)2 + 2CH4
(2) Tf2C(MgBr)2 + TfF → Tf3C(MgBr) + MgBrF
(3) Tf3C(MgBr) + H2SO4 → Tf3CH + MgBrHSO4
In its anionic form, the lanthanide salts of triflidic acid ("triflides") have been shown to be more efficient Lewis acids than the corresponding triflates.[3][4] The triflide anion has also been employed as the anionic component of ionic liquids.[5]
References
- ↑ Barrett, A. G. M.; Braddock, D. C.; Raju, G. S. "Tris[(trifluoromethyl)sulfonyl]methane and Related Salts" Encyclopedia of Reagents for Organic Synthesis". doi:10.1002/047084289X.rn00441
- ↑ Turowsky, Lutz; Seppelt, Konrad (1988-06-01). "Tris[(trifluoromethyl)sulfonyl]methane, HC(SO2CF3)3". Inorganic Chemistry. 27 (12): 2135–2137. doi:10.1021/ic00285a025. ISSN 0020-1669.
- ↑ Waller, Francis J.; Barrett, Anthony G. M.; Braddock, D. Christopher; Ramprasad, Dorai; McKinnell, R. Murray; White, Andrew J. P.; Williams, David J.; Ducray, Richard (1999-04-01). "Tris(trifluoromethanesulfonyl)methide ("Triflide") Anion: Convenient Preparation, X-ray Crystal Structures, and Exceptional Catalytic Activity as a Counterion with Ytterbium(III) and Scandium(III)". The Journal of Organic Chemistry. 64 (8): 2910–2913. doi:10.1021/jo9800917. ISSN 0022-3263.
- ↑ Ishihara, Kazuaki; Hiraiwa, Yukihiro; Yamamoto, Hisashi (2000-01-01). "Homogeneous Debenzylation Using Extremely Active Catalysts: Tris(triflyl)methane, Scandium(III) Tris(triflyl)methide, and Copper(II) Tris(triflyl)methide". Synlett. 2000 (01): 80–82. doi:10.1055/s-2000-6436. ISSN 0936-5214.
- ↑ Johansson, Katarina M.; Adebahr, Josefina; Howlett, Patrick C.; Forsyth, Maria; MacFarlane, Douglas R. (2007-01-01). "N-Methyl-N-Alkylpyrrolidinium Bis(perfluoroethylsulfonyl)amide ([NPf2]) and Tris(trifluoromethanesulfonyl)methide ([CTf3]) Salts: Synthesis and Characterization". Australian Journal of Chemistry. 60 (1): 57–63. doi:10.1071/ch06299.