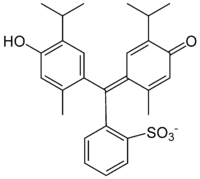
Thymol blue
 | |
 | |
| Names | |
|---|---|
| IUPAC name
4-[9-(4-hydroxy-2-methyl-5-propan- 2-yl-phenyl)-7,7-dioxo-8-oxa- 7λ6-thiabicyclo[4.3.0]nona-1,3,5-trien-9-yl]- 5-methyl-2-propan-2-yl-phenol | |
| Other names
α-hydroxy-α,α-bis(5-hydroxycarvacryl)- o-toluenesulfonic acid γ-sultone; thymolsulfonephthalein | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.000.886 |
PubChem CID |
|
| |
| |
| Properties | |
| C27H30O5S | |
| Molar mass | 466.59 g·mol−1 |
| Appearance | Brownish-green crystal powder |
| Melting point | 221–224 °C (430–435 °F; 494–497 K) decomposes[1] |
| Insoluble | |
| UV-vis (λmax) | 594 nm (1st) 376 nm (2nd)[1] |
| Hazards | |
| Main hazards | Harmful |
EU classification (DSD) (outdated) |
|
| NFPA 704 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Thymol blue (thymolsulphonephthalein) is a brownish-green or reddish-brown crystalline powder that is used as a pH indicator. It is insoluble in water but soluble in alcohol and dilute alkali solutions.
| Thymol blue (pH indicator) | ||
| below pH 8.0 | above pH 9.6 | |
| 8.0 | ⇌ | 9.6 |
| Thymol blue (pH indicator) | ||
| below pH 1.2 | above pH 2.8 | |
| 1.2 | ⇌ | 2.8 |
It transitions from red to yellow at pH 1.2–2.8 and from yellow to blue at pH 8.0–9.6. It is usually a component of Universal indicator.
At wavelength (378 - 382) nm, extinction coefficient > 8000 and at wavelength (298 - 302) nm , the extinction coefficient > 12000.[2]
Structures
Thymol blue has different structures at different pH.
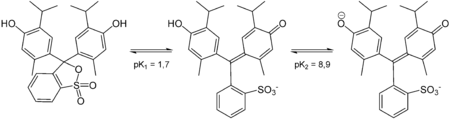

Color of thymol blue solution at different acid-base conditions: left: acidic, middle: neutral, right: alkaline
Safety
It may cause irritation. Its toxicological properties have not been fully investigated. Harmful if swallowed, Acute Toxicity. Only Hazardous when percent values are above 10%.[3]
Bibliography
- Merck. "Thymol Blue." The Merck Index. 14th ed. 2006. Accessed via web on 2007-02-25.
References
- 1 2 Thymol Blue
- ↑ "Product Specification" (PDF). Thymol Blue- ACS reagent. 1: 1. 9 October 2017 – via Product Specification.
- ↑ Pubchem. "Thymol blue". pubchem.ncbi.nlm.nih.gov. Retrieved 2017-10-09.
External links
| Wikimedia Commons has media related to Thymol blue. |
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.
