Texas root rot
| Texas root rot | |
|---|---|
 | |
| Scientific classification | |
| Kingdom: | Fungi |
| Division: | Ascomycota |
| Class: | Pezizomycetes |
| Order: | Pezizales |
| Family: | Rhizinaceae |
| Genus: | Phymatotrichopsis |
| Species: | P. omnivora |
| Binomial name | |
| Phymatotrichopsis omnivora (Duggar) Hennebert, (1973) | |
| Synonyms | |
|
Grandiniella omnivora (Shear) Burds., (1977) | |
Texas root rot (also known as Phymatotrichopsis root rot, Phymatotrichum root rot, cotton root rot, or, in the older literature, Ozonium root rot) is a pathogen fairly common in Mexico and the southwestern United States that causes sudden wilt and death of affected plants, usually during the warmer months. It is a soil-borne fungus of the species Phymatotrichopsis omnivora that attacks the roots of susceptible plants. It was first discovered in 1888, and was named by Duggar in 1916.[1][2]
A monograph of this disease, which includes a historical review, was written by R.B. Streets and H.E. Bloss in 1973.[3]
Host and symptoms
Phymatotrichopsis omnivora is a necrotic fungal pathogen that has a very broad host range, attacking almost 2000 dicotyledonus species. It is known to inhabit in the alkaline, calcareous soils in Southwest United States.[4] It particularly targets dicots as most monocots are immune.[5] Economically important plant host affected by the species include: peanuts, cotton, alfalfa, apple, pecans, and ornamental trees.
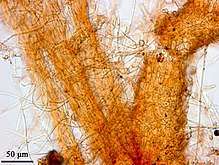
First symptoms of disease is often chlorosis on the leaves. This is then followed by browning and wilting of the leaves. Eventually after two weeks of the first symptoms the plant dies.[4] In the field, infected cotton plants exhibit wilting in the mid to late summer form large circular patches and later die. Upon closer examination, the host plant's vascular system will show extensive discoloration.[4] Underneath the soil, more observable signs are present. Distinctive cuniform branched hyphae are found on infected root tissue which are observable with compound microscope (Figure 1). In addition, taproots of the infect plant will be covered in myclieal strands.[6]
Another macro sign is during favorable high moisture environmental conditions where spore mats appear.[4] Despite the name, the purpose of these spore mats are not known to aid in dispersal. Although presence of the condial phase on the spore mats is known the function of the produced condia remains unknown since condia germination is rarely observed[2] The spore mats are tan and white, found on the soil surface near the infected plant.[4]
Environment
Phymatotrichopsis omnivore thrives in humid warm weather conditions. The disease is most infectious in humid and warm conditions. Plant symptoms are most dramatic in warm conditions as plants become ever more dependent on their roots during these conditions.[5] The pathogen, P. omnivore, also prefers alkane calcareous soils that rarely freeze over, hence why it is mainly restricted to southwest US.[7]
Disease cycle
The disease overwinters as sclerotia or as mycelium on dead plant tissue. Once spring to early summer arrives, germination phase with hyphae growth continues. Following this, root colonization occurs. In Mid and late summer you begin to see the disease at its infectious stage, which is when you start to observe associated symptoms.[2] The pathogen will penetrate the host, and colonize plant root tissue causing root rot. This initially causes the first symptoms of chlorotic leaves and eventually wilting.[2] The pathogen will then eventually disseminate infecting neighboring plants, with infected plant tissue serving as a secondary inoculum fueling the disease. In situation of high moisture, conidia are produced on spore mats but its role in dispersal is currently unknown since very rarely conidia were found to germinate and facilitate the spread of disease.[2]
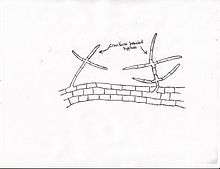
P. omnivore will form several differentiated hyphae. Initially, germ tubes emerge from soil residing sclerotia that overwinter.[2] The sclerotia structure acts as the primary inoculum in affected fields.[8] The pathogen hyphae will either infect the host root or form mycelium with a differentiated rind. Upon contact with host roots, P. omnivore forms a mycelial mantle on the root's surface.[8] This leads to necrosis of epidermis and underlying cortical tissue, leading to root lesions. As the disease progresses, the roots are covered by the characteristic cinnamon-colored mycelial strands covered with acircular sterile hyphae, a diagnostic sign of Texas Root Rot.[8] The roots at later stages of infection show extensive vascular discoloration due to root necroses. The mycelial strand and symptom development in field-infected roots are even more conspicuous on cotton.[8] During the late summer and fall, mycelial strands formed on the root surfaces or in the soil form sclerotia to survive the winter, thus completing the life cycle.
Pathogenesis
As a soil borne pathogen, Phymatotrichopsis omnivore enters the plant host via the roots.[2] It penetrates the host by growing infectious hyphae that cover the host plant root's epidermis and eventually infects epidermis and cortical cell junctions of plant host instead of having specialized penetration organs like an appressoria.[2] From there the fungal pathogen infects root vascular system and begin cause cortical root lesions, which is most pronounced in cotton Microarray analysis and gene expression profiling revealed that certain pathways related to plant defense such as jasmonic acid, ethylene, and flavonoid production were reduced at later infectious stages.[8] This suggests that in order to avoid plant defense Phymatotrichopsis omnivore suppresses the production of these phytochemical defenses to ensure success.[8]
Control
There are currently no successful methods in controlling the severity and occurrence of the disease on a commercial scale [1],.[8] No fungicides and soil fumigation strategies have proved successful.[1] Chemicals such as methyl bromide, anhydrous ammonia and ammonium salts were tested as effective but required deep injective into the soil, therefore expensive and not commercially feasible.[2] In addition, systemic fungicides such as benzimidazoles and sterol biosynthesis inhibitors were shown to limit P. omnivore as well, but systemic fungicides are expensive, and exhibit poor soil penetration therefore pursued cautiously.[9] Currently the best methods are limited to mainly cultural practices. The most effective strategy is identifying diseased plots and planting non-susceptible plants.[1] Another strategy is host escape, by planting early maturing varieties for harvest before summer when the disease is most active6. Also by targeting the sclerotia, the survival structure, using deep plowing of a moldboard plow (6-8 inches deep). There are also some thoughts on biocontrol through microbial competition that may limit P. omnivore, the proposed genera Gliocladia and Trichoderma are thought to be antagonistic to P. omnivore, however these findings are not adequately tested and reported.[2][9]
Importance
The pathogen is particularly economically important pathogen in cotton causing significant losses on average of $100 million annually (based on disease loss estimates and price data for 1980–2008; provided by the National Cotton Council of America, http://www.cotton.org).[2] It is also a serious disease that can cause serious yield loss on many horticultural crops such as peanuts, apples, peaches, and ornamental trees.[2]
References
- 1 2 3 4 Damicone, John P (January 2014). "Phymatotrichum Root Rot" (PDF). Oklahoma Cooperative Extension Service. Retrieved December 1, 2016.
- 1 2 3 4 5 6 7 8 9 10 11 12 UPPALAPATI S.R, YOUNG, C. A., MAREK, S. M. and MYSORE, K. S (2010). "Phymatotrichum (cotton) root rot caused by Phymatotrichopsis omnivora: retrospects and prospects". Molecular Plant Pathology. 11 (3): 325–334. doi:10.1111/j.1364-3703.2010.00616.x – via Wiley Online Library.
- ↑ R.B. Streets and H.E. Bloss. 1973. Phymatotrichum Root Rot. Monograph #8. The American Phytopathological Society, St. Paul, MN
- 1 2 3 4 5 Goldberg, Natalie (2005). "Phymatotrichum Root Rot" (PDF). New Mexico State College of Agriculture, Consumer and Environmental Sciences. Retrieved 17 November 2016.
- 1 2 Olson, Mary (February 2000). "Cotton (Texas) Root Rot". Cooperative Extension, College of Agriculture & Life Sciences, The University of Arizona. Retrieved 1 December 2016.
- ↑ Marek, S.M.; Hansen, K.; Romanish, M.; Thorn, R.G. (2016-12-08). "Molecular systematics of the cotton root rot pathogen, Phymatotrichopsis omnivora". Persoonia. 22: 63–74. doi:10.3767/003158509X430930. ISSN 0031-5850. PMC 2789547. PMID 20198139.
- ↑ Percy, R.G (1983). "Potential range of phymatotrichum omnivorum as determined by edaphic factors" (PDF). Plant Disease. 69 (9): 981–983. doi:10.1094/pd-67-981.
- 1 2 3 4 5 6 7 Uppalapati, S. R.; et al. (2009). "Global Gene Expression Profiling During Medicago truncatula–Phymatotrichopsis omnivora Interaction Reveals a Role for Jasmonic Acid, Ethylene, and the Flavonoid Pathway in Disease Development". Molecular Plant Microbe Interaction. 22: 7–17. doi:10.1094/mpmi-22-1-0007.
- 1 2 7Kenerley, C.M. and Stack, J.P (1987). "Influence of assessment methods on selection of fungal anatagonists of the sclerotium-forming fungus Phymatotrichum omnivorum". Canadian Journal of Microbiology. 33 (7): 632–635. doi:10.1139/m87-110.