Tetramethylammonium pentafluoroxenate
 | |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Tetramethylammonium pentafluoridoxenonate(−) | |||
| Identifiers | |||
| Properties | |||
| N(CH3)4XeF5 | |||
| Molar mass | 300.4308 g/mol | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
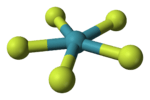
Tetramethylammonium pentafluoroxenate is the chemical compound with the formula N(CH3)4XeF5. The XeF−
5 ion it contains was the first example of a planar pentagonal AX5E2 species.[1] It was prepared by the reaction of N(CH3)4F with xenon tetrafluoride, N(CH3)4F being chosen because it can be prepared in anhydrous form and is readily soluble in organic solvents.[1] The anion is planar, with the fluorine atoms in a slightly distorted pentagonal coordination (Xe–F bond lengths 197.9–203.4 pm, and F–X–F bond angles 71.5–72.3°).[1] Other salts have been prepared with sodium, caesium and rubidium, and vibrational spectra show that these contain the same planar ion.[1] The isolated anion has the unusual point group of D5d.

References
- 1 2 3 4 Christe K. O., Curtis E. C., Dixon D. A., Mercier H. P.,. Sanders J. C. P, Schrobilgen G. J. (1991). "The pentafluoroxenate(IV) anion, XeF5−: the first example of a pentagonal planar AX5 species". J. Am. Chem. Soc. 113 (9): 3351–3361. doi:10.1021/ja00009a021.
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.