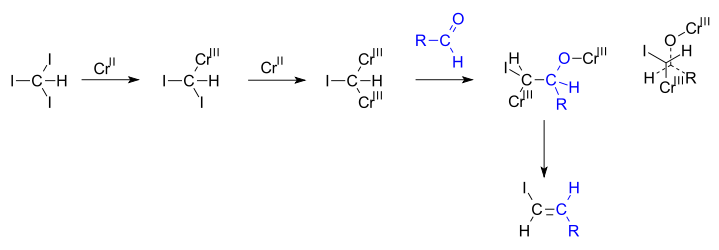
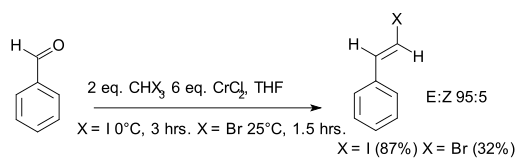Takai olefination
| Takai olefination | |
|---|---|
| Named after | Kazuhiko Takai |
| Reaction type | Carbon-carbon bond forming reaction |
Takai olefination in organic chemistry describes the organic reaction of an aldehyde with a diorganochromium compound to form an alkene. In the original 1986 publication[1] the aldehyde is benzaldehyde and the organochromium species is generated from iodoform or bromoform and an excess of chromium(II) chloride. The reaction product is a vinyl halide. The main selling point is the E-configuration of the double bond. According to the principal investigator Kazuhiko Takai, existing alternatives such as the Wittig reaction only yield mixtures.
In the reaction mechanism proposed by Takai, chromium(II) is oxidized to chromium(III) eliminating two equivalents of a halide. The geminal carbodianion complex thus formed (determined as [Cr2Cl4(CHI)(THF)4])[2][3] reacts with the aldehyde in a 1,2-addition along one of the carbon to chromium bonds and in the next step both chromium bearing groups engage in an elimination reaction. In newman projection it can be seen how the steric bulks of chromium groups and the steric bulks of the alkyl and halogen groups drive this reaction towards anti elimination.[4]
Takai–Utimoto olefination
In a second publication the scope of the reaction was extended to diorganochromium intermediates bearing alkyl groups instead of halogens:[5]
References
- ↑ Takai, K.; Nitta, K.; Utimoto, K. (1986). "Simple and selective method for aldehydes (RCHO) -> (E)-haloalkenes (RCH:CHX) conversion by means of a haloform-chromous chloride system". J. Am. Chem. Soc. 108 (23): 7408–7410. doi:10.1021/ja00283a046.
- ↑ Werner, Daniel; Anwander, Reiner (2018-09-28). "Unveiling the Takai Olefination Reagent via Tris(tert-butoxy)siloxy Variants". Journal of the American Chemical Society. doi:10.1021/jacs.8b08739. ISSN 0002-7863.
- ↑ Murai, Masahito; Taniguchi, Ryuji; Hosokawa, Naoki; Nishida, Yusuke; Mimachi, Hiroko; Oshiki, Toshiyuki; Takai, Kazuhiko (2017-09-05). "Structural Characterization and Unique Catalytic Performance of Silyl-Group-Substituted Geminal Dichromiomethane Complexes Stabilized with a Diamine Ligand". Journal of the American Chemical Society. 139 (37): 13184–13192. doi:10.1021/jacs.7b07487. ISSN 0002-7863.
- ↑ Kürti, László; Czakó, Barbara (2005). Strategic Applications of Named Reactions in Organic Synthesis. Burlington; San Diego; London: Elsevier Academic Press. ISBN 978-0-12-369483-6.
- ↑ Okazoe, T.; Takai, Kazuhiko; Utimoto, K. (1987). "(E)-Selective olefination of aldehydes by means of gem-dichromium reagents derived by reduction of gem-diiodoalkanes with chromium(II) chloride". J. Am. Chem. Soc. 109 (3): 951–953. doi:10.1021/ja00237a081.