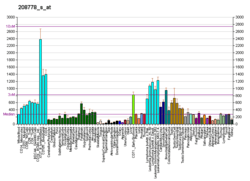
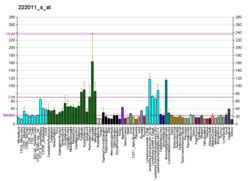
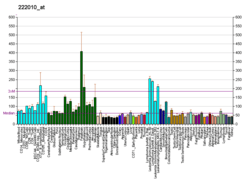
T-complex 1
T-complex protein 1 subunit alpha is a protein that in humans is encoded by the TCP1 gene.[5][6][7]
Function
This gene encodes a molecular chaperone that is a member of the chaperonin containing TCP1 complex (CCT), also known as the TCP1 ring complex (TRiC). This complex consists of two identical stacked rings, each containing eight different proteins. Unfolded polypeptides enter the central cavity of the complex and are folded in an ATP-dependent manner. The complex folds various proteins, including actin and tubulin. Alternate transcriptional splice variants of this gene, encoding different isoforms, have been characterized.[7]
Interactions
T-complex 1 has been shown to interact with PPP4C[8][9] and HDAC3.[10] CCT also directly interacts with lectin type oxidized LDL receptor-1 (LOX-1) while its ligand oxidized low density lipoprotein (OxLDL) disassociates CCT from LOX-1.[11]
References
- 1 2 3 GRCh38: Ensembl release 89: ENSG00000120438 - Ensembl, May 2017
- 1 2 3 GRCm38: Ensembl release 89: ENSMUSG00000068039 - Ensembl, May 2017
- ↑ "Human PubMed Reference:".
- ↑ "Mouse PubMed Reference:".
- ↑ Fonatsch C, Gradl G, Ragoussis J, Ziegler A (Oct 1987). "Assignment of the TCP1 locus to the long arm of human chromosome 6 by in situ hybridization". Cytogenet Cell Genet. 45 (2): 109–12. doi:10.1159/000132439. PMID 3476253.
- ↑ Willison K, Kelly A, Dudley K, Goodfellow P, Spurr N, Groves V, Gorman P, Sheer D, Trowsdale J (Nov 1987). "The human homologue of the mouse t-complex gene, TCP1, is located on chromosome 6 but is not near the HLA region". EMBO J. 6 (7): 1967–74. PMC 553584. PMID 3653076.
- 1 2 "Entrez Gene: TCP1 t-complex 1".
- ↑ Chen GI, Tisayakorn S, Jorgensen C, D'Ambrosio LM, Goudreault M, Gingras AC (Oct 2008). "PP4R4/KIAA1622 Forms a Novel Stable Cytosolic Complex with Phosphoprotein Phosphatase 4". J. Biol. Chem. 283 (43): 29273–84. doi:10.1074/jbc.M803443200. PMC 2662017. PMID 18715871.
- ↑ Gingras AC, Caballero M, Zarske M, Sanchez A, Hazbun TR, Fields S, Sonenberg N, Hafen E, Raught B, Aebersold R (Nov 2005). "A novel, evolutionarily conserved protein phosphatase complex involved in cisplatin sensitivity". Mol. Cell. Proteomics. 4 (11): 1725–40. doi:10.1074/mcp.M500231-MCP200. PMID 16085932.
- ↑ Guenther MG, Yu J, Kao GD, Yen TJ, Lazar MA (Dec 2002). "Assembly of the SMRT–histone deacetylase 3 repression complex requires the TCP-1 ring complex". Genes Dev. 16 (24): 3130–5. doi:10.1101/gad.1037502. PMC 187500. PMID 12502735.
- ↑ Bakthavatsalam D, Soung RH, Tweardy DJ, Chiu W, Dixon RA, Woodside DG (Jun 2014). "Chaperonin-containing TCP-1 complex directly binds to the cytoplasmic domain of the LOX-1 receptor". FEBS Lett. 588 (13): 2133–40. doi:10.1016/j.febslet.2014.04.049. PMC 4100626. PMID 24846140.
Further reading
- Horwich AL, Willison KR (1993). "Protein folding in the cell: functions of two families of molecular chaperone, hsp 60 and TF55-TCP1". Philos. Trans. R. Soc. Lond. B Biol. Sci. 339 (1289): 313–25, discussion 325–6. doi:10.1098/rstb.1993.0030. PMID 8098536.
- Burston SG, Clarke AR (1997). "Molecular chaperones: physical and mechanistic properties". Essays Biochem. 29: 125–36. PMID 9189717.
- Blanché H, Wright LG, Vergnaud G, de Gouyon B, Lauthier V, Silver LM, Dausset J, Cann HM, Spielman RS (1992). "Genetic mapping of three human homologues of murine t-complex genes localizes TCP10 to 6q27, 15 cM distal to TCP1 and PLG". Genomics. 12 (4): 826–8. doi:10.1016/0888-7543(92)90317-L. PMID 1572657.
- Dawson SJ, White LA (1992). "Treatment of Haemophilus aphrophilus endocarditis with ciprofloxacin". J. Infect. 24 (3): 317–20. doi:10.1016/S0163-4453(05)80037-4. PMID 1602151.
- Yaffe MB, Farr GW, Miklos D, Horwich AL, Sternlicht ML, Sternlicht H (1992). "TCP1 complex is a molecular chaperone in tubulin biogenesis". Nature. 358 (6383): 245–8. doi:10.1038/358245a0. PMID 1630491.
- Lewis VA, Hynes GM, Zheng D, Saibil H, Willison K (1992). "T-complex polypeptide-1 is a subunit of a heteromeric particle in the eukaryotic cytosol". Nature. 358 (6383): 249–52. doi:10.1038/358249a0. PMID 1630492.
- Ursic D, Culbertson MR (1991). "The yeast homolog to mouse Tcp-1 affects microtubule-mediated processes". Mol. Cell. Biol. 11 (5): 2629–40. PMC 360032. PMID 1901944.
- Kirchhoff C, Willison K (1990). "Nucleotide and amino-acid sequence of human testis-derived TCP1". Nucleic Acids Res. 18 (14): 4247. doi:10.1093/nar/18.14.4247. PMC 331189. PMID 2377466.
- Roobol A, Holmes FE, Hayes NV, Baines AJ, Carden MJ (1995). "Cytoplasmic chaperonin complexes enter neurites developing in vitro and differ in subunit composition within single cells". J. Cell Sci. 108 (4): 1477–88. PMID 7615668.
- Ashworth A (1994). "Two acetyl-CoA acetyltransferase genes located in the t-complex region of mouse chromosome 17 partially overlap the Tcp-1 and Tcp-1x genes". Genomics. 18 (2): 195–8. doi:10.1006/geno.1993.1454. PMID 7904580.
- Miklos D, Caplan S, Mertens D, Hynes G, Pitluk Z, Kashi Y, Harrison-Lavoie K, Stevenson S, Brown C, Barrell B (1994). "Primary structure and function of a second essential member of the heterooligomeric TCP1 chaperonin complex of yeast, TCP1 beta". Proc. Natl. Acad. Sci. U.S.A. 91 (7): 2743–7. doi:10.1073/pnas.91.7.2743. PMC 43446. PMID 7908441.
- Chen X, Sullivan DS, Huffaker TC (1994). "Two yeast genes with similarity to TCP-1 are required for microtubule and actin function in vivo". Proc. Natl. Acad. Sci. U.S.A. 91 (19): 9111–5. doi:10.1073/pnas.91.19.9111. PMC 44757. PMID 7916460.
- Kubota H, Hynes G, Carne A, Ashworth A, Willison K (1994). "Identification of six Tcp-1-related genes encoding divergent subunits of the TCP-1-containing chaperonin". Curr. Biol. 4 (2): 89–99. doi:10.1016/S0960-9822(94)00024-2. PMID 7953530.
- Frydman J, Hartl FU (1996). "Principles of chaperone-assisted protein folding: differences between in vitro and in vivo mechanisms". Science. 272 (5267): 1497–502. doi:10.1126/science.272.5267.1497. PMID 8633246.
- Moudjou M, Bordes N, Paintrand M, Bornens M (1996). "gamma-Tubulin in mammalian cells: the centrosomal and the cytosolic forms". J. Cell Sci. 109 (4): 875–87. PMID 8718679.
- Morrison K, Papapetrou C, Attwood J, Hol F, Lynch SA, Sampath A, Hamel B, Burn J, Sowden J, Stott D, Mariman E, Edwards YH (1997). "Genetic mapping of the human homologue (T) of mouse T(Brachyury) and a search for allele association between human T and spina bifida". Hum. Mol. Genet. 5 (5): 669–74. doi:10.1093/hmg/5.5.669. PMID 8733136.
- Masuno M, Fukao T, Song XQ, Yamaguchi S, Orii T, Kondo N, Imaizumi K, Kuroki Y (1997). "Assignment of the human cytosolic acetoacetyl-coenzyme A thiolase (ACAT2) gene to chromosome 6q25.3-q26". Genomics. 36 (1): 217–8. doi:10.1006/geno.1996.0452. PMID 8812443.