Sodium thiosulfate (medical use)
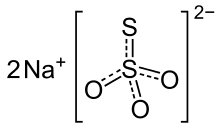 Sodium thiosulfate, structural formula | |
| Clinical data | |
|---|---|
| Trade names | Versiclear, others |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| Pregnancy category |
|
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| Chemical and physical data | |
| Formula | Na2S2O3 |
| Molar mass | 158.108 |
| 3D model (JSmol) | |
| |
| |
Sodium thiosulfate, also spelled sodium thiosulphate, is used as a medication to treat cyanide poisoning, pityriasis versicolor, and to decrease side effects from cisplatin.[1][2] For cyanide poisoning it is often used after the medication sodium nitrite and typically only recommended for severe cases.[1][3] It is either given by injection into a vein or applied to the skin.[1]
Side effects may include vomiting, joint pain, mood changes, psychosis, and ringing in the ears.[2] Safety; however, has not been well studied.[4] It is unclear if use in pregnancy is safe for the baby.[2] Use at the same time in the same intravensous line as hydroxocobalamin is not recommended.[3] In cyanide poisoning sodium nitrite creates methemoglobinemia which removes cyanide from mitochondria.[3] Sodium thiosulfate then binds with cyanide creating the nontoxic thiocyanate.[3]
Sodium thiosulfate came into medical use for cyanide poisoning in the 1930s.[5] It is on the World Health Organization's List of Essential Medicines, the most effective and safe medicines needed in a health system.[6] The cost in the United States per dose as of 2013 is about 20 USD while together with sodium nitrite it costs 110 USD.[7]
Medical uses
The main use of sodium thiosulfate is in cyanide poisoning and pityriasis versicolor.[1]
Cyanide poisoning
In cyanide poisoning there are concerns that sodium thiosulfate may not have a fast enough onset of action to be very useful without the additional use of other agents.[8]
In those who have both cyanide poisoning and carbon monoxide poisoning sodium thiosulfate by itself is recommended.[9]
Other
There is also a small amount of evidence supporting its use in calciphylaxis, where blood vessels calcify.[10]
References
- 1 2 3 4 WHO Model Formulary 2008 (PDF). World Health Organization. 2009. pp. 66, 298. ISBN 9789241547659. Archived (PDF) from the original on 13 December 2016. Retrieved 8 January 2017.
- 1 2 3 "Sodium thiosulfate Intravenous Advanced Patient Information - Drugs.com". www.drugs.com. Archived from the original on 18 January 2017. Retrieved 15 January 2017.
- 1 2 3 4 "Sodium Thiosulfate Solution for Injection - Summary of Product Characteristics (SPC) - (eMC)". www.medicines.org.uk. Archived from the original on 16 January 2017. Retrieved 15 January 2017.
- ↑ "Sodium Thiosulfate Injection - FDA prescribing information, side effects and uses". www.drugs.com. Archived from the original on 18 January 2017. Retrieved 15 January 2017.
- ↑ Dart, Richard C. (2004). Medical Toxicology. Lippincott Williams & Wilkins. p. 172. ISBN 9780781728454. Archived from the original on 2017-01-16.
- ↑ "WHO Model List of Essential Medicines (19th List)" (PDF). World Health Organization. April 2015. Archived (PDF) from the original on 13 December 2016. Retrieved 8 December 2016.
- ↑ Gasco, L; Rosbolt, MB; Bebarta, VS (April 2013). "Insufficient stocking of cyanide antidotes in US hospitals that provide emergency care". Journal of Pharmacology & Pharmacotherapeutics. 4 (2): 95–102. PMID 23761707.
- ↑ Hall, AH; Dart, R; Bogdan, G (June 2007). "Sodium thiosulfate or hydroxocobalamin for the empiric treatment of cyanide poisoning?". Annals of Emergency Medicine. 49 (6): 806–13. PMID 17098327.
- ↑ Baren, Jill M. (2008). Pediatric Emergency Medicine. Elsevier Health Sciences. p. 1018. ISBN 978-1416000877. Archived from the original on 2017-01-16.
- ↑ Auriemma, M; Carbone, A; Di Liberato, L; Cupaiolo, A; Caponio, C; De Simone, C; Tulli, A; Bonomini, M; Amerio, P (1 October 2011). "Treatment of cutaneous calciphylaxis with sodium thiosulfate: two case reports and a review of the literature". American Journal of Clinical Dermatology. 12 (5): 339–46. PMID 21834598.