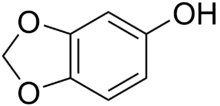
Sesamol
 | |
| Names | |
|---|---|
| Preferred IUPAC name
2H-1,3-Benzodioxol-5-ol | |
| Other names
1,3-Benzodioxol-5-ol Benzo[d][1,3]dioxol-5-ol Sesamol 3,4-Methylenedioxyphenol | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.007.784 |
| KEGG | |
PubChem CID |
|
| |
| |
| Properties | |
| C7H6O3 | |
| Molar mass | 138.12 g/mol |
| Melting point | 62 to 65 °C (144 to 149 °F; 335 to 338 K) |
| Boiling point | 121 to 127 °C (250 to 261 °F; 394 to 400 K) at 5 mmHg |
| Hazards | |
| NFPA 704 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Sesamol is a natural organic compound which is a component of sesame seeds and sesame oil. It is a white crystalline solid that is a derivative of phenol. It is sparingly soluble in water, but miscible with most oils. It can be produced by organic synthesis from heliotropine.
Sesamol has been found to be an antioxidant that may prevent the spoilage of oils,[2][3] It also may prevent the spoilage of oils by acting as an antifungal.[4]
Sesame oil is used in Ayurvedic Medicine.[5]
It can be used in the synthesis of paroxetine.[6]:138-141
See also
References
- ↑ Sesamol Archived 2010-01-14 at the Wayback Machine. at Chemicalland21.com
- ↑ Joo Yeon Kim, Dong Seong Choi and Mun Yhung Jung "Antiphoto-oxidative Activity of Sesamol in Methylene Blue- and Chlorophyll-Sensitized Photo-oxidation of Oil" J. Agric. Food Chem., 51 (11), 3460 -3465, 2003.
- ↑ Ohsawa, Toshiko. "Sesamol and sesaminol as antioxidants." New Food Industry (1991), 33(6), 1-5.
- ↑ Wynn, James P.; Kendrick, Andrew; Ratledge, Colin. "Sesamol as an inhibitor of growth and lipid metabolism in Mucor circinelloides via its action on malic enzyme." Lipids (1997), 32(6), 605-610.
- ↑ "A Closer Look at Ayurvedic Medicine". Focus on Complementary and Alternative Medicine. Bethesda, Maryland: National Center for Complementary and Alternative Medicine (NCCAM), US National Institutes of Health (NIH). 12 (4). Fall 2005 – Winter 2006. Archived from the original on 2006-12-09.
- ↑ Li, Jie Jack (2004). Contemporary drug synthesis. Hoboken, N.J.: Wiley. ISBN 978-0-471-21480-9.
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.
