Sericin
Sericin is a protein created by Bombyx mori (silkworms) in the production of silk.[1][2]
Silk is a fibre produced by the silkworm in production of its cocoon. It consists mainly of two proteins, fibroin and sericin. Silk consists of 70–80% fibroin and 20–30% sericin; fibroin being the structural center of the silk, and sericin being the gum coating the fibres and allowing them to stick to each other.
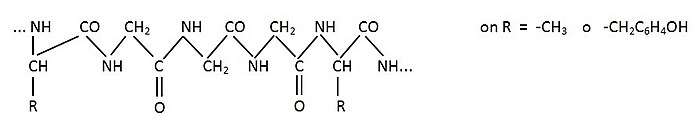
Composed structurally of 18 different amino acids, and 32% serine, in most commonly, a randomized amorphous coil. When in the amorphous coil, sericin can also be easily be converted into a β-sheet conformation, via repeated moisture absorption and mechanical stretching. Using gamma ray examination, it was determined that sericin fibers are composed typically of three layers, all with fibers running in different patterns of directionality. The innermost layer, typically is composed of longitudinally running fibers, the middle layer is composed of cross fiber directional patterned fibers, and the outer layer consists of fiber directional fibers. The overall structure can also vary based on temperature, whereas the lower the temperature, there were typically more β-sheet conformations than random amorphous coils. There are also three different types of sericin, which make up the layers found on top of the fibrin. Sericin A, which is insoluble in water, is the outermost layer, and contains approximately 17% nitrogen, along with amino acids such as serine, threonine, aspartic acid, and glycine. Sericin B, composed the middle layer and is nearly the same as sericin A, but also contains tryptophan. Sericin C is the innermost layer, the layer that comes closest to and is adjacent to fibroin. Also insoluble in water, sericin C can be separated from the fibroin via the addition of a hot, weak acid. Sericin C also contains the amino acids present in B, along with the addition of proline.
Sericin has also been used in medicine and cosmetics. Due to its elasticity and tensile strength, along with a natural affinity for keratin, sericin is primarily used in medicine for wound suturing. It also has a natural infection resistance, and is used variably due to excellent biocompatibility, and thus is used commonly as a wound coagulant as well. When used in cosmetics, sericin has been found to improve skin elasticity and several anti-aging factors, including an anti-wrinkle property. This is done by minimizing water loss from the skin. To determine this, scientists ran several experimental procedures, including a hydroxyproline assay, impedance measurements, water loss from the epidermis and scanning electron microscopy to analyze the rigidity and dryness of the skin. The presence or sericin increases hydroxyproline in the stratum corneum, which in turn, decrease skin impedance, thus increasing skin moisture. Adding in pluronic and carbopol, two other factors that can be included in sericin gels, perform the action of repairing natural moisture factors (NMF), along with minimizing water loss, and in turn, improving skin moisture.[2]
The chemical composition of sericin is C30H40N10O16. The structure of the sericin is not entirely clear or confirmed, but in a Japanese study conducted in 2007/2008 by Mayer, S. and Maric, M. suggested the following:[3]
See also Murat Ersel, Yigit Uyanikgil, Funda Karbek Akarca, Emel Oyku Cetin Uyanikgil et al.: Effects of Silk Sericin on Incision Wound Healing in a Dorsal Skin Flap Wound Healing Rat Model, Conference Paper of 10th European Congress On Emergency Medicine Eusem At Vienna, Austria, Oct. 2016.
References
- ↑ "Sericin". Cytokines and Cells Online Pathfinder Encyclopedia. January 2008. Retrieved 27 April 2012.
- 1 2 M N Padamwar; A P Pawar (April 2004). "Silk sericin and its applications: A review" (PDF). Journal of Scientific & Industrial Research. 63 (4): 323–329.
- ↑ Mayer, S. Maric, M.: Preparation of silk protein sericin as mitogenic factor for better mammalian cell culture., Dept. of applied Chemistry and Biotechnology, University of Fukui, Japan, 2008
- ↑ "The Protein Model Portal". 2015-10-26.